Cellular and molecular basis of pulmonary arterial hypertension
- PMID: 19555855
- PMCID: PMC2790324
- DOI: 10.1016/j.jacc.2009.04.018
Cellular and molecular basis of pulmonary arterial hypertension
Abstract
Pulmonary arterial hypertension (PAH) is caused by functional and structural changes in the pulmonary vasculature, leading to increased pulmonary vascular resistance. The process of pulmonary vascular remodeling is accompanied by endothelial dysfunction, activation of fibroblasts and smooth muscle cells, crosstalk between cells within the vascular wall, and recruitment of circulating progenitor cells. Recent findings have reestablished the role of chronic vasoconstriction in the remodeling process. Although the pathology of PAH in the lung is well known, this article is concerned with the cellular and molecular processes involved. In particular, we focus on the role of the Rho family guanosine triphosphatases in endothelial function and vasoconstriction. The crosstalk between endothelium and vascular smooth muscle is explored in the context of mutations in the bone morphogenetic protein type II receptor, alterations in angiopoietin-1/TIE2 signaling, and the serotonin pathway. We also review the role of voltage-gated K(+) channels and transient receptor potential channels in the regulation of cytosolic [Ca(2+)] and [K(+)], vasoconstriction, proliferation, and cell survival. We highlight the importance of the extracellular matrix as an active regulator of cell behavior and phenotype and evaluate the contribution of the glycoprotein tenascin-c as a key mediator of smooth muscle cell growth and survival. Finally, we discuss the origins of a cell type critical to the process of pulmonary vascular remodeling, the myofibroblast, and review the evidence supporting a contribution for the involvement of endothelial-mesenchymal transition and recruitment of circulating mesenchymal progenitor cells.
Conflict of interest statement
Dr. Adnot has indicated no conflict of interest to disclose.
Dr. Archer has received grant support from the National Institutes of Health.
Dr. Dupuis has served as a consultant for Actelion Pharmaceuticals, Pfizer, and Encysive Technologies.
Dr. Jones has received an honorarium from Novartis.
Dr. MacLean has received funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and the British Heart Foundation (BHF).
Dr. McMurtry has received a research grant from the National Heart, Lung and Blood Institute.
Dr. Morrell has received a research grant from Novartis and has received honoraria for educational lectures from Actelion Pharmaceuticals, GlaxoSmithKline, and Pfizer.
Dr. Stenmark has indicated no conflict of interest to disclose.
Dr. Thistlethwaite has received grant support from the National Institutes of Health and the Center for Medical Research and Education Fund (CMREF).
Dr. Weir has indicated no conflict of interest to disclose.
Dr. Weissmann received a research grant from the Deutsche Forschungsgemeinschaft (DFG) “Excellence Cluster Cardio-Pulmonary System (ECCPS).”
Dr. Yuan has received grant support from the National Institutes of Health.
Figures
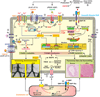
References
-
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. - PubMed
-
- Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. - PubMed
-
- Csortos C, Kolosova I, Verin AD. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the PPP family. Am J Physiol Lung Cell Mol Physiol. 2007;293:L843–L854. - PubMed
-
- Wojciak-Stothard B, Tsang LY, Paleolog E, Hall SM, Haworth SG. Rac1 and RhoA as regulators of endothelial phenotype and barrier function in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1173–L1182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

