Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier
- PMID: 19556244
- PMCID: PMC2755710
- DOI: 10.1074/jbc.M109.033332
Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier
Abstract
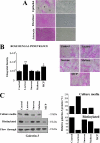
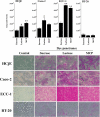
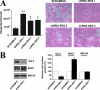
Maintenance of an intact mucosal barrier is critical to preventing damage to and infection of wet-surfaced epithelia. The mechanism of defense has been the subject of much investigation, and there is evidence now implicating O-glycosylated mucins on the epithelial cell surface. Here we investigate a new role for the carbohydrate-binding protein galectin-3 in stabilizing mucosal barriers through its interaction with mucins on the apical glycocalyx. Using the surface of the eye as a model system, we found that galectin-3 colocalized with two distinct membrane-associated mucins, MUC1 and MUC16, on the apical surface of epithelial cells and that both mucins bound to galectin-3 affinity columns in a galactose-dependent manner. Abrogation of the mucin-galectin interaction in four different mucosal epithelial cell types using competitive carbohydrate inhibitors of galectin binding, beta-lactose and modified citrus pectin, resulted in decreased levels of galectin-3 on the cell surface with concomitant loss of barrier function, as indicated by increased permeability to rose bengal diagnostic dye. Similarly, down-regulation of mucin O-glycosylation using a stable tetracycline-inducible RNA interfering system to knockdown c1galt1 (T-synthase), a critical galactosyltransferase required for the synthesis of core 1 O-glycans, resulted in decreased cell surface O-glycosylation, reduced cell surface galectin-3, and increased epithelial permeability. Taken together, these results suggest that galectin-3 plays a key role in maintaining mucosal barrier function through carbohydrate-dependent interactions with cell surface mucins.
Figures




References
-
- Childers N. K., Bruce M. G., McGhee J. R. (1989) Annu. Rev. Microbiol. 43,503–536 - PubMed
-
- Gipson I. K., Argueso P., Beuerman R., Bonini S., Butovich I., Dana R., Dartt D., Gamache D., Ham B., Jumblatt M., Korb D., Kruse F., Ogawa Y., Paulsen F., Stern M., Sweeney D. F., Tiffany J., Ubels J., Willcox M. (2007) Ocul. Surf. 5,179–193 - PubMed
-
- Davies D. E. (2001) Curr. Allergy Asthma Rep. 1,127–133 - PubMed
-
- Einerhand A. W., Renes I. B., Makkink M. K., van der Sluis M., Büller H. A., Dekker J. (2002) Eur. J. Gastroenterol. Hepatol. 14,757–765 - PubMed
-
- Hollingsworth M. A., Swanson B. J. (2004) Nat. Rev. Cancer 4,45–60 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

