Complement inhibitor eculizumab in atypical hemolytic uremic syndrome
- PMID: 19556379
- PMCID: PMC2723971
- DOI: 10.2215/CJN.01090209
Complement inhibitor eculizumab in atypical hemolytic uremic syndrome
Abstract
Background and objectives: Atypical hemolytic uremic syndrome (aHUS) is associated with a congenital or acquired dysregulation of the complement alternative pathway that leads to continuous complement activation on host cells causing inflammation and damage. Eculizumab, a humanized mAb against complement protein C5, inhibits activation of the terminal complement pathway.
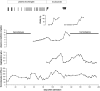
Design, setting, participants, & measurements: We report an adolescent with relapsing unclassified aHUS. On admission, a high plasma creatinine level indicated a poor prognosis, and hemodialysis had to be started. Plasma exchanges were initially effective against the microangiopathic hemolytic activity and allowed a temporary improvement of renal function with termination of hemodialysis after 7 wk. Subsequently, plasma exchanges (three times per week) failed to prevent ongoing aHUS activity and progressive renal failure. After 12 wk, aHUS treatment was switched to eculizumab.
Results: Eculizumab was effective in terminating the microangiopathic hemolytic process in two aHUS relapses; however, after normalization of complement activity, aHUS recurred and ultimately led to anuric end-stage renal failure.
Conclusions: In this patient, complement inhibition by eculizumab temporarily terminated the microangiopathic hemolytic activity. Nevertheless, renal damage as a result of preceding and subsequent aHUS activity resulted in end-stage renal failure; therefore, therapeutic success may depend on early administration of eculizumab. The optimal duration of treatment may be variable and remains to be determined.
Figures
References
-
- Noris M, Remuzzi G: Hemolytic uremic syndrome. J Am Soc Nephrol 16: 1035–1050,2005 - PubMed
-
- Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, Rizzoni G, Taylor CM, Van De Kar N, Zimmerhackl LBEuropean Paediatric Research Group for HUS: A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int 70: 423–431,2006 - PubMed
-
- Kavanagh D, Richards A, Atkinson J: Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med 59: 293–309,2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous