Growth factors, matrices, and forces combine and control stem cells
- PMID: 19556500
- PMCID: PMC2847855
- DOI: 10.1126/science.1171643
Growth factors, matrices, and forces combine and control stem cells
Abstract
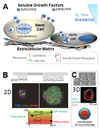
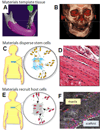
Stem cell fate is influenced by a number of factors and interactions that require robust control for safe and effective regeneration of functional tissue. Coordinated interactions with soluble factors, other cells, and extracellular matrices define a local biochemical and mechanical niche with complex and dynamic regulation that stem cells sense. Decellularized tissue matrices and synthetic polymer niches are being used in the clinic, and they are also beginning to clarify fundamental aspects of how stem cells contribute to homeostasis and repair, for example, at sites of fibrosis. Multifaceted technologies are increasingly required to produce and interrogate cells ex vivo, to build predictive models, and, ultimately, to enhance stem cell integration in vivo for therapeutic benefit.
Figures

References
References and Notes
-
- Pittenger M, Martin B. Circ. Res. 2004;95:9. - PubMed
-
- NIH. 2009. http://clinicaltrials.gov.
-
- Alper J. Nat. Biotechnol. 2009;27:213. - PubMed
-
- Macchiarini P, et al. Lancet. 2008;372:2023.
-
- Amariglio N, et al. PLoS Med. 2009;6:221.
Additional References in Figure Legends
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

