Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase
- PMID: 19556511
- PMCID: PMC2764269
- DOI: 10.1126/science.1171716
Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase
Abstract
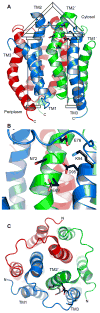
Escherichia coli diacylglycerol kinase (DAGK) represents a family of integral membrane enzymes that is unrelated to all other phosphotransferases. We have determined the three-dimensional structure of the DAGK homotrimer with the use of solution nuclear magnetic resonance. The third transmembrane helix from each subunit is domain-swapped with the first and second transmembrane segments from an adjacent subunit. Each of DAGK's three active sites resembles a portico. The cornice of the portico appears to be the determinant of DAGK's lipid substrate specificity and overhangs the site of phosphoryl transfer near the water-membrane interface. Mutations to cysteine that caused severe misfolding were located in or near the active site, indicating a high degree of overlap between sites responsible for folding and for catalysis.
Figures
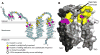
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

