Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis
- PMID: 19556518
- PMCID: PMC2753790
- DOI: 10.1164/rccm.200903-0322OC
Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis
Abstract
Rationale: Lung fibroblasts are key mediators of fibrosis resulting in accumulation of excessive interstitial collagen and extracellular matrix, but their origins are not well defined.
Objectives: We aimed to elucidate the contribution of lung epithelium-derived fibroblasts via epithelial-mesenchymal transition (EMT) in the intratracheal bleomycin model.
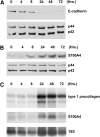
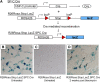
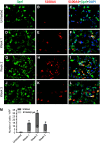
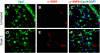
Methods: Primary type II alveolar epithelial cells were cultured from Immortomice and exposed to transforming growth factor-beta(1) and epidermal growth factor. Cell fate reporter mice that permanently mark cells of lung epithelial lineage with beta-galactosidase were developed to study EMT, and bone marrow chimeras expressing green fluorescent protein under the control of the fibroblast-associated S100A4 promoter were generated to examine bone marrow-derived fibroblasts. Mice were given intratracheal bleomycin (0.08 unit). Immunostaining was performed for S100A4, beta-galactosidase, green fluorescent protein, and alpha-smooth muscle actin.
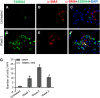
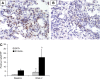
Measurements and main results: In vitro, primary type II alveolar epithelial cells undergo phenotypic changes of EMT when exposed to transforming growth factor-beta(1) and epidermal growth factor with loss of prosurfactant protein C and E-cadherin and gain of S100A4 and type I procollagen. In vivo, using cell fate reporter mice, approximately one-third of S100A4-positive fibroblasts were derived from lung epithelium 2 weeks after bleomycin administration. From bone marrow chimera studies, one-fifth of S100A4-positive fibroblasts were derived from bone marrow at this same time point. Myofibroblasts rarely derived from EMT or bone marrow progenitors.
Conclusions: Both EMT and bone marrow progenitors contribute to S100A4-positive fibroblasts in bleomycin-induced lung fibrosis. However, neither origin is a principal contributor to lung myofibroblasts.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

