Trim24 targets endogenous p53 for degradation
- PMID: 19556538
- PMCID: PMC2710642
- DOI: 10.1073/pnas.0813177106
Trim24 targets endogenous p53 for degradation
Abstract
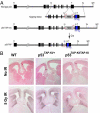
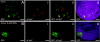
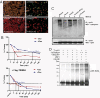
Numerous studies focus on the tumor suppressor p53 as a protector of genomic stability, mediator of cell cycle arrest and apoptosis, and target of mutation in 50% of all human cancers. The vast majority of information on p53, its protein-interaction partners and regulation, comes from studies of tumor-derived, cultured cells where p53 and its regulatory controls may be mutated or dysfunctional. To address regulation of endogenous p53 in normal cells, we created a mouse and stem cell model by knock-in (KI) of a tandem-affinity-purification (TAP) epitope at the endogenous Trp-53 locus. Mass spectrometry of TAP-purified p53-complexes from embryonic stem cells revealed Tripartite-motif protein 24 (Trim24), a previously unknown partner of p53. Mutation of TRIM24 homolog, bonus, in Drosophila led to apoptosis, which could be rescued by p53-depletion. These in vivo analyses establish TRIM24/bonus as a pathway that negatively regulates p53 in Drosophila. The Trim24-p53 link is evolutionarily conserved, as TRIM24 depletion in human breast cancer cells caused p53-dependent, spontaneous apoptosis. We found that Trim24 ubiquitylates and negatively regulates p53 levels, suggesting Trim24 as a therapeutic target to restore tumor suppression by p53.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
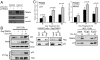
Comment in
-
TRIMming p53 for ubiquitination.Proc Natl Acad Sci U S A. 2009 Jul 14;106(28):11431-2. doi: 10.1073/pnas.0905997106. Epub 2009 Jul 7. Proc Natl Acad Sci U S A. 2009. PMID: 19584248 Free PMC article. No abstract available.
References
-
- Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–724. - PubMed
-
- Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. - PubMed
-
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. - PubMed
-
- Levine AJ, Momand J, Finlay CA. The p53 tumor suppressor gene. Nature. 1991;351:453–456. - PubMed
-
- Poyurovsky MV, Prives C. Unleashing the power of p53: Lessons from mice and men. Genes Dev. 2006;20:125–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

