High fat feeding induces hepatic fatty acid elongation in mice
- PMID: 19557132
- PMCID: PMC2699051
- DOI: 10.1371/journal.pone.0006066
High fat feeding induces hepatic fatty acid elongation in mice
Abstract
Background: High-fat diets promote hepatic lipid accumulation. Paradoxically, these diets also induce lipogenic gene expression in rodent liver. Whether high expression of these genes actually results in an increased flux through the de novo lipogenic pathway in vivo has not been demonstrated.
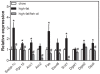
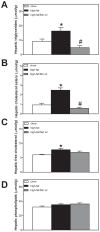
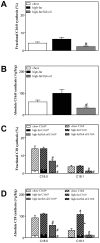
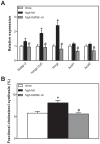
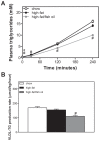
Methodology/principal findings: To interrogate this apparent paradox, we have quantified de novo lipogenesis in C57Bl/6J mice fed either chow, a high-fat or a n-3 polyunsaturated fatty acid (PUFA)-enriched high-fat diet. A novel approach based on mass isotopomer distribution analysis (MIDA) following 1-(13)C acetate infusion was applied to simultaneously determine de novo lipogenesis, fatty acid elongation as well as cholesterol synthesis. Furthermore, we measured very low density lipoprotein-triglyceride (VLDL-TG) production rates. High-fat feeding promoted hepatic lipid accumulation and induced the expression of lipogenic and cholesterogenic genes compared to chow-fed mice: induction of gene expression was found to translate into increased oleate synthesis. Interestingly, this higher lipogenic flux (+74 microg/g/h for oleic acid) in mice fed the high-fat diet was mainly due to an increased hepatic elongation of unlabeled palmitate (+66 microg/g/h) rather than to elongation of de novo synthesized palmitate. In addition, fractional cholesterol synthesis was increased, i.e. 5.8+/-0.4% vs. 8.1+/-0.6% for control and high fat-fed animals, respectively. Hepatic VLDL-TG production was not affected by high-fat feeding. Partial replacement of saturated fat by fish oil completely reversed the lipogenic effects of high-fat feeding: hepatic lipogenic and cholesterogenic gene expression levels as well as fatty acid and cholesterol synthesis rates were normalized.
Conclusions/significance: High-fat feeding induces hepatic fatty acid synthesis in mice, by chain elongation and subsequent desaturation rather than de novo synthesis, while VLDL-TG output remains unaffected. Suppression of lipogenic fluxes by fish oil prevents from high fat diet-induced hepatic steatosis in mice.
Conflict of interest statement
Figures
References
-
- Westerbacka J, Lammi K, Hakkinen AM, Rissanen A, Salminen I, et al. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90:2804–2809. - PubMed
-
- van der Meer RW, Hammer S, Lamb HJ, Frolich M, Diamant M, et al. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab. 2008;93:2702–2708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

