The structure of a biologically active influenza virus ribonucleoprotein complex
- PMID: 19557158
- PMCID: PMC2695768
- DOI: 10.1371/journal.ppat.1000491
The structure of a biologically active influenza virus ribonucleoprotein complex
Abstract
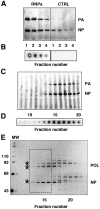
The influenza viruses contain a segmented, single-stranded RNA genome of negative polarity. Each RNA segment is encapsidated by the nucleoprotein and the polymerase complex into ribonucleoprotein particles (RNPs), which are responsible for virus transcription and replication. Despite their importance, information about the structure of these RNPs is scarce. We have determined the three-dimensional structure of a biologically active recombinant RNP by cryo-electron microscopy. The structure shows a nonameric nucleoprotein ring (at 12 Angstrom resolution) with two monomers connected to the polymerase complex (at 18 Angstrom resolution). Docking the atomic structures of the nucleoprotein and polymerase domains, as well as mutational analyses, has allowed us to define the interactions between the functional elements of the RNP and to propose the location of the viral RNA. Our results provide the first model for a functional negative-stranded RNA virus ribonucleoprotein complex. The structure reported here will serve as a framework to generate a quasi-atomic model of the molecular machine responsible for viral RNA synthesis and to test new models for virus RNA replication and transcription.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
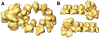
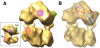





References
-
- Wright PF, Neumann G, Kawaoka Y. Orthomyxoviridae. In: Knipe DM, Howley PM, editors. Fields Virology 5th edition. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1691–1740.
-
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. - PubMed
-
- Murti KG, Webster RG, Jones IM. Localization of RNA polymerases of influenza viral ribonucleoproteins by immunogold labeling. Virology. 1988;164:562–566. - PubMed
-
- Palese P, Shaw M. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology 5th edition. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1647–1689.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

