Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner
- PMID: 19557177
- PMCID: PMC2698216
- DOI: 10.1371/journal.pone.0006049
Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner
Retraction in
-
Retraction: Tbx1 Regulates the BMP-Smad1 Pathway in a Transcription Independent Manner.PLoS One. 2020 Mar 19;15(3):e0230974. doi: 10.1371/journal.pone.0230974. eCollection 2020. PLoS One. 2020. PMID: 32191773 Free PMC article. No abstract available.
Abstract
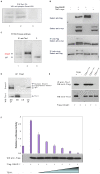
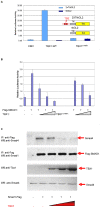
Tbx1 is a T-box transcription factor implicated in DiGeorge syndrome. The molecular function of Tbx1 is unclear although it can transactivate reporters with T-box binding elements. We discovered that Tbx1 binds Smad1 and suppresses the Bmp4/Smad1 signaling. Tbx1 interferes with Smad1 to Smad4 binding, and a mutation of Tbx1 that abolishes transactivation, does not affect Smad1 binding nor does affect the ability to suppress Smad1 activity. In addition, a disease-associated mutation of TBX1 that does not prevent transactivation, prevents the TBX1-SMAD1 interaction. Expression of Tbx1 in transgenic mice generates phenotypes similar to those associated with loss of a Bmp receptor. One phenotype could be rescued by transgenic Smad1 expression. Our data indicate that Tbx1 interferes with Bmp/Smad1 signaling and provide strong evidence that a T-box transcription factor has functions unrelated to transactivation.
Conflict of interest statement
Figures


References
-
- Naiche LA, Harrelson Z, Kelly RG, Papaioannou VE. T-box genes in vertebrate development. Annu Rev Genet. 2005;39:219–239. - PubMed
-
- Stennard FA, Harvey RP. T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development. 2005;132:4897–4910. - PubMed
-
- Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

