Adenosine Monophosphate-Activated Protein Kinase (AMPK) as a New Target for Antidiabetic Drugs: A Review on Metabolic, Pharmacological and Chemical Considerations
- PMID: 19557293
- PMCID: PMC2712919
- DOI: 10.1900/RDS.2009.6.13
Adenosine Monophosphate-Activated Protein Kinase (AMPK) as a New Target for Antidiabetic Drugs: A Review on Metabolic, Pharmacological and Chemical Considerations
Abstract
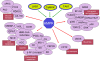
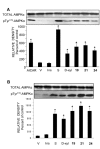
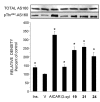
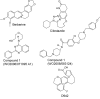
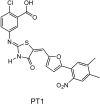
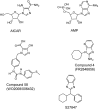
In view of the epidemic nature of type 2 diabetes and the substantial rate of failure of current oral antidiabetic drugs the quest for new therapeutics is intensive. The adenosine monophosphate-activated protein kinase (AMPK) is an important regulatory protein for cellular energy balance and is considered a master switch of glucose and lipid metabolism in various organs, especially in skeletal muscle and liver. In skeletal muscles, AMPK stimulates glucose transport and fatty acid oxidation. In the liver, it augments fatty acid oxidation and decreases glucose output, cholesterol and triglyceride synthesis. These metabolic effects induced by AMPK are associated with lowering blood glucose levels in hyperglycemic individuals. Two classes of oral antihyperglycemic drugs (biguanidines and thiazolidinediones) have been shown to exert some of their therapeutic effects by directly or indirectly activating AMPK. However, side effects and an acquired resistance to these drugs emphasize the need for the development of novel and efficacious AMPK activators. We have recently discovered a new class of hydrophobic D-xylose derivatives that activates AMPK in skeletal muscles in a non insulin-dependent manner. One of these derivatives (2,4;3,5-dibenzylidene-D-xylose-diethyl-dithioacetal) stimulates the rate of hexose transport in skeletal muscle cells by increasing the abundance of glucose transporter-4 (GLUT-4) in the plasma membrane through activation of AMPK. This compound reduces blood glucose levels in diabetic mice and therefore offers a novel strategy of therapeutic intervention strategy in type 2 diabetes. The present review describes various classes of chemically-related compounds that activate AMPK by direct or indirect interactions and discusses their potential for candidate antihyperglycemic drug development.
Figures
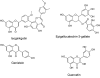
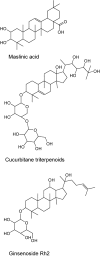
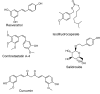
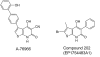
References
-
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546(1):113–120. - PubMed
-
- Thornton C, Snowden MA, Carling D. Identification of a novel AMP-activated protein kinase beta subunit isoform that is highly expressed in skeletal muscle. J Biol Chem. 1998;273(20):12443–12450. - PubMed
-
- Amodeo GA, Rudolph MJ, Tong L. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1. Nature. 2007;449(7161):492–495. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
