Pattern formation in the Drosophila eye disc
- PMID: 19557685
- PMCID: PMC2713679
- DOI: 10.1387/ijdb.072483jr
Pattern formation in the Drosophila eye disc
Abstract
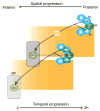
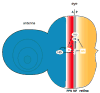
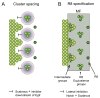
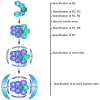
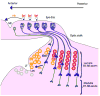
Differentiation of the Drosophila compound eye from the eye imaginal disc is a progressive process: columns of cells successively differentiate in a posterior to anterior sequence, clusters of cells form at regularly spaced intervals within each column, and individual photoreceptors differentiate in a defined order within each cluster. The progression of differentiation across the eye disc is driven by a positive autoregulatory loop of expression of the secreted molecule Hedgehog, which is temporally delayed by the intercalation of a second signal, Spitz. Hedgehog refines the spatial position at which each column initiates its differentiation by inducing secondary signals that act over different ranges to control the expression of positive and negative regulators. The position of clusters within each column is controlled by secreted inhibitory signals from clusters in the preceding column, and a single founder neuron, R8, is singled out within each cluster by Notch-mediated lateral inhibition. R8 then sequentially recruits surrounding cells to differentiate by producing a short-range signal, Spitz, which induces a secondary short-range signal, Delta. Intrinsic transcription factors act in combination with these two signals to produce cell-type diversity within the ommatidium. The Hedgehog and Spitz signals are transported along the photoreceptor axons and reused within the brain as long-range and local cues to trigger the differentiation and assembly of target neurons.
Figures
Comment in
-
Pattern formation today.Int J Dev Biol. 2009;53(5-6):653-8. doi: 10.1387/ijdb.082594cc. Int J Dev Biol. 2009. PMID: 19557673 Free PMC article. Review.
References
-
- APIDIANAKIS Y, GRBAVEC D, STIFANI S, DELIDAKIS C. Groucho mediates a ci-independent mechanism of hedgehog repression in the anterior wing pouch. Development. 2001;128:4361–70. - PubMed
-
- ARTAVANIS-TSAKONAS S, RAND MD, LAKE RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–6. - PubMed
-
- AZA-BLANC P, RAMIREZ-WEBER FA, LAGET MP, SCHWARTZ C, KORNBERG TB. Proteolysis that is inhibited by Hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–53. - PubMed
-
- BAKER NE, MLODZIK M, RUBIN GM. Spacing differentiation in the developing Drosophila eye: A fibrinogen-related lateral inhibitor encoded by scabrous. Science. 1990;250:1370–7. - PubMed
-
- BAKER NE, YU S, HAN D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr Biol. 1996;6:1290–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

