Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality
- PMID: 19557755
- PMCID: PMC2722958
- DOI: 10.1002/anie.200806273
Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality
Abstract
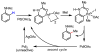
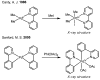
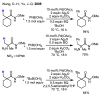
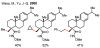
In the past decade, palladium-catalyzed C-H activation/C-C bond-forming reactions have emerged as promising new catalytic transformations; however, development in this field is still at an early stage compared to the state of the art in cross-coupling reactions using aryl and alkyl halides. This Review begins with a brief introduction of four extensively investigated modes of catalysis for forming C-C bonds from C-H bonds: Pd(II)/Pd(0), Pd(II)/Pd(IV), Pd(0)/Pd(II)/Pd(IV), and Pd(0)/Pd(II) catalysis. A more detailed discussion is then directed towards the recent development of palladium(II)-catalyzed coupling of C-H bonds with organometallic reagents through a Pd(II)/Pd(0) catalytic cycle. Despite the progress made to date, improving the versatility and practicality of this new reaction remains a tremendous challenge.
Figures
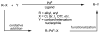
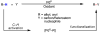


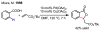

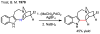


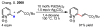

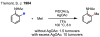

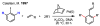
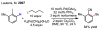
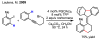
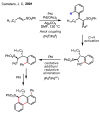

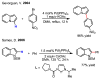
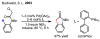
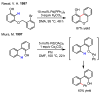
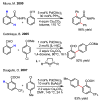
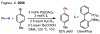

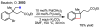


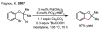

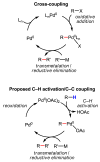



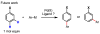
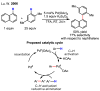
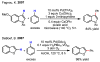

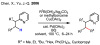
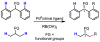
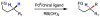
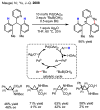
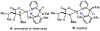
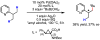
References
-
-
For reviews, see: Stille JK. Angew Chem Int Ed. 1986;25:508.Heck RF. In: Comprehensive Organic Synthesis. 4. Trost BM, Fleming I, editors. Pergamon; Oxford: 1991. p. 833.Miyaura N, Suzuki A. Chem Rev. 1995;95:2457.Diederich F, Stang PJ, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; New York: 1998. Luh TY, Leung MK, Wong KT. Chem Rev. 2000;100:3187.Hiyama T. J Organomet Chem. 2002;653:58.E-i Negishi, Hu Q, Huang Z, Qian M, Wang G. Aldrichimica Acta. 2005;38:71.Trost BM, Crawley ML. Chem Rev. 2003;103:2921.Nicolaou KC, Bulger PG, Sarlah D. Angew Chem Int Ed. 2005;44:4442.Surry DS, Buchwald SL. Angew Chem Int Ed. 2008;47:6338.Hartwig JF. Nature. 2008;455:314.Denmark SE, Regens CS. Acc Chem Res. 2008;41:1486.
-
-
-
For ligand developments, see: Old DW, Wolfe JP, Buchwald SL. J Am Chem Soc. 1998;120:9722.Littke AF, Fu GC. Angew Chem Int Ed. 1998;37:3387.Herrmann WA, Reisinger CP, Spiegler M. J Organomet Chem. 1998;557:93.Hamann BC, Hartwig JF. J Am Chem Soc. 1998;120:7369.Zhang C, Huang J, Trudell ML, Nolan SP. J Org Chem. 1999;64:3804.Rataboul F, Zapf A, Jackstell R, Harkal S, Riermeier T, Monsees A, Dingerdissen U, Beller M. Chem Eur J. 2004;10:2983.
-
-
-
For reviews on C–H activation chemistry, see: Crabtree RH. Chem Rev. 1985;85:245.Shilov AE, Shul’pin GB. Chem Rev. 1997;97:2879.Stahl SS, Labinger JA, Bercaw JE. Angew Chem Int Ed. 1998;37:2181.Bergman RG. Nature. 2007;446:391.Brookhart M, Green MLH, Parkin G. Proc Natl Acad Sci. 2007;104:6908.
-
-
-
For comprehensive reviews on cyclopalladation reactions, see: Ryabov AD. Synthesis. 1985:233.Canty AJ. In: Comprehensive organometallic chemistry II: a review of the literature 1982–1994. Abel EW, Stone FGA, Wilkinson G, editors. Pergamon; 1995. pp. 225–255.
-
-
-
a) For an early example of cyclopalladation of sp2 C-H bonds directed by azo groups, see: Cope AC, Siekman RW. J Am Chem Soc. 1965;87:3272.b) For an early example of cyclopalladation of sp3 C–H bonds directed by oximes see: Constable AG, McDonald WS, Sawkins LC, Shaw BL. J Chem Soc Chem Commun. 1978:1061.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

