The G18V CRYGS mutation associated with human cataracts increases gammaS-crystallin sensitivity to thermal and chemical stress
- PMID: 19558189
- PMCID: PMC2735583
- DOI: 10.1021/bi900467a
The G18V CRYGS mutation associated with human cataracts increases gammaS-crystallin sensitivity to thermal and chemical stress
Abstract
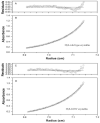
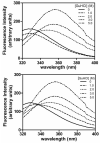
GammaS-crystallin, important in maintaining lens transparency, is a monomeric betagamma-crystallin comprising two paired homologous domains, each with two Greek key motifs. An autosomal dominant cortical progressive cataract has been associated with a G18V mutation in human gammaS-crystallin. To investigate the molecular mechanism of this cataract and confirm the causative nature of the G18V mutation, we examined resultant changes in conformation and stability. Human gammaS-crystallin cDNA was cloned into pET-20b(+), and the G18V mutant was generated by site-directed mutagenesis. Recombinant HgammaS-crystallins were expressed in Escherichia coli and purified by ion-exchange and size-exclusion chromatography. By analytical ultracentrifugation wild-type and mutant HgammaS-crystallins are monomers of about 21.95 +/- 0.21 and 20.89 +/- 0.18 kDa, respectively, and have similar secondary structures by far-UV CD. In increasing levels of guanidine hydrochloride (GuHCl), a sharp red shift in fluorescence lambda(max) and increase in emission correlating with exposure of tryptophans to the protein surface are detected earlier in the mutant protein. Under thermal stress, the G18V mutant begins to show changes in tryptophan fluorescence above 42 degrees C and shows a Tm of 65 degrees C as monitored by CD at 218 nm, while wild-type HgammaS-crystallin is very stable with Tm values of 75.5 and 75.0 degrees C as measured by fluorescence and CD, respectively. Equilibrium unfolding/refolding experiments as a function of GuHCl confirm the relative instability of the G18V mutant. Wild-type HgammaS-crystallin exhibits a two-state transition and reversible refolding above 1.0 M GuHCl, but the unfolding transition of mutant HgammaS-crystallin shows an intermediate state. The first transition (N --> I) shows a [GuHCl](1/2) of 0.5 M while the second transition (I --> U) has the same [GuHCl](1/2) as wild-type HgammaS-crystallin, about 2.0 M. Our present study confirms the high stability of wild-type HgammaS-crystallin and demonstrates that the G18V mutation destabilizes the protein toward heat and GuHCl-induced unfolding. These biophysical characteristics are consistent with the progressive cataract formation seen in the family members carrying this mutation.
Figures





Similar articles
-
Human γS-Crystallin Mutation F10_Y11delinsLN in the First Greek Key Pair Destabilizes and Impairs Tight Packing Causing Cortical Lamellar Cataract.Int J Mol Sci. 2023 Sep 20;24(18):14332. doi: 10.3390/ijms241814332. Int J Mol Sci. 2023. PMID: 37762633 Free PMC article.
-
Human αB-crystallin discriminates between aggregation-prone and function-preserving variants of a client protein.Biochim Biophys Acta Gen Subj. 2020 Mar;1864(3):129502. doi: 10.1016/j.bbagen.2019.129502. Epub 2019 Dec 5. Biochim Biophys Acta Gen Subj. 2020. PMID: 31812542 Free PMC article.
-
Cataract-causing G18V eliminates the antagonization by ATP against the crowding-induced destabilization of human γS-crystallin.Biochem Biophys Res Commun. 2020 Sep 24;530(3):554-560. doi: 10.1016/j.bbrc.2020.07.070. Epub 2020 Aug 1. Biochem Biophys Res Commun. 2020. PMID: 32753316
-
Gamma crystallins of the human eye lens.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):333-43. doi: 10.1016/j.bbagen.2015.06.007. Epub 2015 Jun 25. Biochim Biophys Acta. 2016. PMID: 26116913 Review.
-
Potential role of βB1 crystallin in cataract formation:a systematic review.Arch Biochem Biophys. 2025 Aug;770:110463. doi: 10.1016/j.abb.2025.110463. Epub 2025 May 10. Arch Biochem Biophys. 2025. PMID: 40355021
Cited by
-
Cataract-causing defect of a mutant γ-crystallin proceeds through an aggregation pathway which bypasses recognition by the α-crystallin chaperone.PLoS One. 2012;7(5):e37256. doi: 10.1371/journal.pone.0037256. Epub 2012 May 24. PLoS One. 2012. PMID: 22655036 Free PMC article.
-
The mutation V42M distorts the compact packing of the human gamma-S-crystallin molecule, resulting in congenital cataract.PLoS One. 2012;7(12):e51401. doi: 10.1371/journal.pone.0051401. Epub 2012 Dec 21. PLoS One. 2012. PMID: 23284690 Free PMC article.
-
Exploring the aggregation propensity of γS-crystallin protein variants using two-dimensional spectroscopic tools.J Phys Chem B. 2013 Nov 21;117(46):14294-301. doi: 10.1021/jp408000k. Epub 2013 Nov 12. J Phys Chem B. 2013. PMID: 24219230 Free PMC article.
-
Protein misfolding and aggregation in cataract disease and prospects for prevention.Trends Mol Med. 2012 May;18(5):273-82. doi: 10.1016/j.molmed.2012.03.005. Epub 2012 Apr 19. Trends Mol Med. 2012. PMID: 22520268 Free PMC article. Review.
-
Human γS-Crystallin Mutation F10_Y11delinsLN in the First Greek Key Pair Destabilizes and Impairs Tight Packing Causing Cortical Lamellar Cataract.Int J Mol Sci. 2023 Sep 20;24(18):14332. doi: 10.3390/ijms241814332. Int J Mol Sci. 2023. PMID: 37762633 Free PMC article.
References
-
- Lubsen NH, Aarts HJM, Schoenmakers JGG. The evolution of lenticular proteins: the beta- and gamma-crystallin supergene family. Prog. Biophys. Mol. Biol. 1988;51:47–76. - PubMed
-
- Blundell T, Lindley P, Miller L, Moss D, Slingsby C, Tickle I, Turnell B, Wistow G. The molecular structure and stability of the eye lens: x-ray analysis of gamma-crystallin II. Nature. 1981;289:771–777. - PubMed
-
- Shimeld SM, Purkiss AG, Dirks RP, Bateman OA, Slingsby C, Lubsen NH. Urochordate betagamma-crystallin and the evolutionary origin of the vertebrate eye lens. Curr. Biol. 2005;15:1684–1689. - PubMed
-
- Bloemendal H, de JW, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004;86:407–485. - PubMed
-
- Sinha D, Esumi N, Jaworski C, Kozak CA, Pierce E, Wistow G. Cloning and mapping the mouse Crygs gene and non-lens expression of gammaS-crystallin. Mol. Vis. 1998;4:8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

