(-)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis
- PMID: 19558547
- PMCID: PMC11158506
- DOI: 10.1111/j.1349-7006.2009.01241.x
(-)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis
Abstract
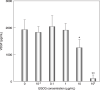
The receptor tyrosine kinase vascular endothelial growth factor (VEGF) receptor (VEGFR) plays an important role in tumor angiogenesis of hepatocellular carcinoma (HCC). (-)-Epigallocatechin gallate (EGCG), the major biologically active component of green tea, inhibits growth in a variety of human cancer cells by inhibiting the activation of several types of receptor tyrosine kinases. In this study, we examined the effects of EGCG on the activity of the VEGF-VEGFR axis in human HCC cells. The levels of total and phosphorylated (i.e. activated) form of VEGFR-2 protein (p-VEGFR-2) were observed to increase in a series of human HCC cell lines in comparison to the Hc normal human hepatocytes. EGCG preferentially inhibited the growth of HuH7 HCC cells, which express constitutive activation of the VEGF-VEGFR axis, in comparison to Hc cells. Treatment of HuH7 cells with EGCG caused a time- and dose-dependent decrease in the expression of VEGFR-2 and p-VEGFR-2 proteins. The production of VEGF from HuH7 cells was reduced by treatment with EGCG. Drinking of EGCG significantly inhibited the growth of HuH7 xenografts in nude mice and this was associated with inhibition of the activation of VEGFR-2 and its related downstream signaling molecules, including ERK and Akt. EGCG drinking also decreased the expression of Bcl-x(L) protein and VEGF mRNA in the xenografts. These findings suggest that EGCG can exert, at least in part, its growth-inhibitive effect on HCC cells by inhibiting the VEGF-VEGFR axis. EGCG might therefore be useful in the treatment of HCC.
Figures

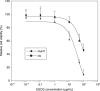

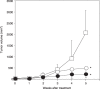
 ); group 2, 0.01% EGCG‐drinking group (
); group 2, 0.01% EGCG‐drinking group ( ); and group 3, 0.1% EGCG‐drinking group (
); and group 3, 0.1% EGCG‐drinking group ( ). The growth curve of HuH7 tumors in each group are represented. Bars, SD. *P < 0.05: significant differences obtained by comparison with EGCG‐untreated control group.
). The growth curve of HuH7 tumors in each group are represented. Bars, SD. *P < 0.05: significant differences obtained by comparison with EGCG‐untreated control group.
References
-
- El‐Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–76. - PubMed
-
- Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology 2007; 72(Suppl 1): 30–44. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V et al . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

