Efficacy of sulphadoxine-pyrimethamine with or without artesunate for the treatment of uncomplicated Plasmodium falciparum malaria in southern Mozambique: a randomized controlled trial
- PMID: 19558654
- PMCID: PMC2709115
- DOI: 10.1186/1475-2875-8-141
Efficacy of sulphadoxine-pyrimethamine with or without artesunate for the treatment of uncomplicated Plasmodium falciparum malaria in southern Mozambique: a randomized controlled trial
Abstract
Background: An artemisinin-based combination therapy, artesunate (AS) plus sulphadoxine-pyrimethamine (SP), was compared to SP monotherapy to provide evidence of further treatment options in southern Mozambique.
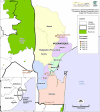
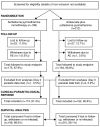
Methods: Between 2003 and 2005, 411 patients over one year and 10 kg with uncomplicated Plasmodium falciparum malaria were randomly allocated SP (25/1.25 mg per kg day 0) or AS/SP (as above plus 4 mg/kg artesunate days 0, 1 and 2). Allocation was concealed, but treatment was open-label except to microscopists. The primary objective was the relative risk of treatment failure, which was assessed using World Health Organization response definitions modified to a 42-day follow-up.
Results: Of the 411 subjects enrolled, 359 (87.3%) completed the follow up period (SP n = 175, AS/SP n = 184). A survival analysis including 408 subjects showed that the polymerase chain reaction-adjusted cure rates were 90.4% (95% confidence interval [CI] 84.9%-93.9%) and 98.0% (95% CI 94.8%-99.3%) for SP and AS/SP respectively. Multivariable analysis showed that treatment with AS/SP decreased the relative hazard of treatment failure by 80% compared to SP (hazard ratio [HR] 0.2; 95% CI 0.1-0.6) and age over seven years decreased the relative hazard of failure by 70% (HR 0.3; 95% CI 0.1-0.9), when compared to younger age. However, having a quintuple dhfr/dhps mutation increased the relative hazard of failure compared to fewer mutations (HR 3.2; 95% CI 1.3-7.5) and baseline axillary temperature increased the relative hazard of failure by 50% for each degree C increase (HR 1.5; 95% CI 1.1-2.2).
Conclusion: While both treatments were efficacious, AS plus SP significantly decreased the relative hazard of treatment failure compared to SP monotherapy Artesunate plus sulphadoxine-pyrimethamine, but not sulphadoxine-pyrimethamine monotherapy, met the current WHO criteria of >95% efficacy for policy implementation.
Trial registration: NCT00203736 and NCT00203814.
Figures


Similar articles
-
Five years of large-scale dhfr and dhps mutation surveillance following the phased implementation of artesunate plus sulfadoxine-pyrimethamine in Maputo Province, Southern Mozambique.Am J Trop Med Hyg. 2010 May;82(5):788-94. doi: 10.4269/ajtmh.2010.09-0401. Am J Trop Med Hyg. 2010. PMID: 20439956 Free PMC article.
-
Extended high efficacy of the combination sulphadoxine-pyrimethamine with artesunate in children with uncomplicated falciparum malaria on the Benin coast, West Africa.Malar J. 2009 Mar 3;8:37. doi: 10.1186/1475-2875-8-37. Malar J. 2009. PMID: 19257898 Free PMC article. Clinical Trial.
-
Adding artesunate to sulphadoxine-pyrimethamine greatly improves the treatment efficacy in children with uncomplicated falciparum malaria on the coast of Benin, West Africa.Malar J. 2007 Dec 21;6:170. doi: 10.1186/1475-2875-6-170. Malar J. 2007. PMID: 18154655 Free PMC article. Clinical Trial.
-
Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2019 Jan 8;1(1):CD006404. doi: 10.1002/14651858.CD006404.pub3. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD006404. doi: 10.1002/14651858.CD006404.pub4. PMID: 30620055 Free PMC article. Updated.
-
[Combined antimalarial therapy using artemisinin].Parassitologia. 2004 Jun;46(1-2):85-7. Parassitologia. 2004. PMID: 15305693 Review. Italian.
Cited by
-
Five years of large-scale dhfr and dhps mutation surveillance following the phased implementation of artesunate plus sulfadoxine-pyrimethamine in Maputo Province, Southern Mozambique.Am J Trop Med Hyg. 2010 May;82(5):788-94. doi: 10.4269/ajtmh.2010.09-0401. Am J Trop Med Hyg. 2010. PMID: 20439956 Free PMC article.
-
Assessment of the Feasibility, Acceptability, and Impact of Implementing Seasonal Malaria Chemoprevention in Nampula Province, Mozambique: Protocol for a Hybrid Effectiveness-Implementation Study.JMIR Res Protoc. 2021 Sep 15;10(9):e27855. doi: 10.2196/27855. JMIR Res Protoc. 2021. PMID: 34524109 Free PMC article.
-
Impact of dhps mutations on sulfadoxine-pyrimethamine protective efficacy and implications for malaria chemoprevention.Nat Commun. 2025 May 8;16(1):4268. doi: 10.1038/s41467-025-58326-z. Nat Commun. 2025. PMID: 40341172 Free PMC article.
-
Molecular surveillance of Plasmodium falciparum drug resistance markers reveals partial recovery of chloroquine susceptibility but sustained sulfadoxine-pyrimethamine resistance at two sites of different malaria transmission intensities in Rwanda.Acta Trop. 2016 Dec;164:329-336. doi: 10.1016/j.actatropica.2016.09.008. Epub 2016 Sep 17. Acta Trop. 2016. PMID: 27647575 Free PMC article.
-
Mapping Antimalarial Drug Resistance in Mozambique: A Systematic Review of Plasmodium falciparum Genetic Markers Post-ACT Implementation.Int J Mol Sci. 2024 Dec 20;25(24):13645. doi: 10.3390/ijms252413645. Int J Mol Sci. 2024. PMID: 39769406 Free PMC article.
References
-
- World Health Organization, Regional Office for Africa. Country health system fact sheet 2006 Mozambique http://www.who.int/countries/moz/moz/en/
-
- Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77:181–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

