Early MRI response monitoring of patients with advanced hepatocellular carcinoma under treatment with the multikinase inhibitor sorafenib
- PMID: 19558720
- PMCID: PMC2714320
- DOI: 10.1186/1471-2407-9-208
Early MRI response monitoring of patients with advanced hepatocellular carcinoma under treatment with the multikinase inhibitor sorafenib
Abstract
Background: New therapeutic principles in clinical oncology require the adjustment of response criteria to govern therapy decisions. For advanced hepatocellular carcinoma (HCC) a new era has recently begun by the approval of the multikinase inhibitor sorafenib. As a unique feature, HCC usually develops in a diseased liver and current imaging technologies employing classical response criteria have not been prospectively evaluated for this new treatment.
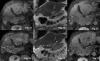
Methods: MRI signal patterns were assessed in 21 advanced HCC patients receiving sorafenib. MRI was performed at baseline and in short-term intervals thereafter. Signal changes under therapy on T1WI, T2WI and post-gadolinium images including necrosis volume and its ratio to the entire tumor volume were compared to baseline imaging. To assess the association between the categorical variables, Fisher's exact tests were applied for a statistical analysis. Survey time ranged from 2-65 weeks, and a total of 39 target lesions were evaluated.
Results: Signal abnormalities during sorafenib therapy were disclosed by T1WI and T2WI in 15/21 patients. The predominant tumor signal change was hyperintensity on both T1WI and T2WI. Interestingly, most patients developed MRI signal changes within 4 weeks of therapy; in contrast, two non-responders did not show any signal alteration at follow-up. Under therapy, 16/21 patients presented with new or progressive necrosis, whereas 7 patients achieved temporarily >75% tumor necrosis under sorafenib. Significantly associated MRI variables were increase in T1WI signal and tumor necrosis (p = 0.017) as well as increase of tumor necrosis with an elevated ratio of necrotic to vital tumor areas (p = 0.002). Remarkably, some (3/13) of the patients developing necrotic tumor areas showed a relevant (>20%) increase in tumor volume, which should be considered in the assessment of imaging studies.
Conclusion: As sorafenib induces early intralesional necrosis with profound changes in T1WI/T2WI MRI signal intensities and measurable necrotic tumor areas in most HCC patients, early MRI-based evaluation could pave the way for its rationale and cost-effective application.
Figures


References
-
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. - DOI - PubMed
-
- Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

