NF-kappaB activity in endothelial cells is modulated by cell substratum interactions and influences chemokine-mediated adhesion of natural killer cells
- PMID: 19558775
- PMCID: PMC2857529
- DOI: 10.3727/096368909788534979
NF-kappaB activity in endothelial cells is modulated by cell substratum interactions and influences chemokine-mediated adhesion of natural killer cells
Abstract
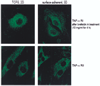
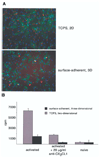
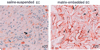
Because changes in subendothelial matrix composition are associated with alterations of the endothelial immune phenotype, we sought to understand if cytokine-induced NF-kappaB activity and downstream effects depend on substrate adherence of endothelial cells (EC). We compared the upstream phosphorylation cascade, activation of NF-kappaB, and expression/secretion of downstream effects of EC grown on tissue culture polystyrene plates (TCPS) with EC embedded within collagen-based matrices (MEEC). Adhesion of natural killer (NK) cells was quantified in vitro and in vivo. NF-kappaB subunit p65 nuclear levels were significantly lower and p50 significantly higher in cytokine-stimulated MEEC than in EC-TCPS. Despite similar surface expression of TNF-alpha receptors, MEEC had significantly decreased secretion and expression of IL-6, IL-8, MCP-1, VCAM-1, and ICAM-1. Attenuated fractalkine expression and secretion in MEEC (two to threefold lower than in EC-TCPS; p < 0.0002) correlated with 3.7-fold lower NK cell adhesion to EC (6,335 +/- 420 vs. 1,735 +/- 135 cpm; p < 0.0002). Furthermore, NK cell infiltration into sites of EC implantation in vivo was significantly reduced when EC were embedded within matrix. Matrix embedding enables control of EC substratum interaction. This in turn regulates chemokine and surface molecule expression and secretion, in particular of those compounds within NF-kappaB pathways, chemoattraction of NK cells, local inflammation, and tissue repair.
Figures





Similar articles
-
Thrombin-induced expression of endothelial CX3CL1 potentiates monocyte CCL2 production and transendothelial migration.J Leukoc Biol. 2008 Jul;84(1):215-23. doi: 10.1189/jlb.0907652. Epub 2008 Apr 24. J Leukoc Biol. 2008. PMID: 18436581
-
Membrane-associated lymphotoxin on natural killer cells activates endothelial cells via an NF-kappaB-dependent pathway.Transplantation. 1998 Nov 15;66(9):1211-9. doi: 10.1097/00007890-199811150-00017. Transplantation. 1998. PMID: 9825820
-
[Effect of Huoxue injection on oxidized low-density lipoprotein induced activation of human umbilical vein endothelial cells].Zhongguo Zhong Yao Za Zhi. 2008 Jul;33(13):1617-21. Zhongguo Zhong Yao Za Zhi. 2008. PMID: 18837329 Chinese.
-
Adenoviral-mediated overexpression of I(kappa)B(alpha) in endothelial cells inhibits natural killer cell-mediated endothelial cell activation.Transplantation. 1996 Oct 15;62(7):967-72. doi: 10.1097/00007890-199610150-00016. Transplantation. 1996. PMID: 8878392
-
Subendothelial resistin enhances monocyte transmigration in a co-culture of human endothelial and smooth muscle cells by mechanisms involving fractalkine, MCP-1 and activation of TLR4 and Gi/o proteins signaling.Int J Biochem Cell Biol. 2014 May;50:29-37. doi: 10.1016/j.biocel.2014.01.022. Epub 2014 Feb 6. Int J Biochem Cell Biol. 2014. PMID: 24508784 Review.
Cited by
-
CD34+ circulating cells display signs of immune activation in patients with acute coronary syndrome.Heart Vessels. 2018 Dec;33(12):1559-1569. doi: 10.1007/s00380-018-1220-7. Epub 2018 Jul 12. Heart Vessels. 2018. PMID: 30003322
-
Cell matrix contact modifies endothelial major histocompatibility complex class II expression in high-glucose environment.Am J Physiol Heart Circ Physiol. 2013 Dec 1;305(11):H1592-9. doi: 10.1152/ajpheart.00018.2013. Epub 2013 Sep 16. Am J Physiol Heart Circ Physiol. 2013. PMID: 24043258 Free PMC article.
-
Fractalkine/CX3CL1 in Neoplastic Processes.Int J Mol Sci. 2020 May 25;21(10):3723. doi: 10.3390/ijms21103723. Int J Mol Sci. 2020. PMID: 32466280 Free PMC article. Review.
-
Tuning of collagen scaffold properties modulates embedded endothelial cell regulatory phenotype in repair of vascular injuries in vivo.Adv Healthc Mater. 2015 Oct 28;4(15):2220-8. doi: 10.1002/adhm.201500457. Epub 2015 Sep 1. Adv Healthc Mater. 2015. PMID: 26333178 Free PMC article.
-
Abrocitinib Attenuates Microglia-Mediated Neuroinflammation after Traumatic Brain Injury via Inhibiting the JAK1/STAT1/NF-κB Pathway.Cells. 2022 Nov 13;11(22):3588. doi: 10.3390/cells11223588. Cells. 2022. PMID: 36429017 Free PMC article.
References
-
- Aznar-Salatti J, Escolar G, Cases A, Gomez-Ortiz G, Anton P, Castillo R, Revert L, Ordinas A. Uraemic medium causes endothelial cell dysfunction characterized by an alteration of the properties of its subendothelial matrix. Nephrol. Dial. Transplant. 1995;10(12):2199–2204. - PubMed
-
- Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ. Evidence for NK cell subsets based on chemokine receptor expression. J. Immunol. 2006;177(11):7833–7840. - PubMed
-
- Borlongan CV, Thanos CG, Skinner SJ, Geaney M, Emerich DF. Transplants of encapsulated rat choroid plexus cells exert neuroprotection in a rodent model of Huntington’s disease. Cell Transplant. 2007;16(10):987–992. - PubMed
-
- Cao G, Lu Y, Gao R, Xin Y, Teng D, Wang J, Li Y. Expression of fractalkine, CX3CR1, and vascular endothelial growth factor in human chronic renal allograft rejection. Transplant. Proc. 2006;38(7):1998–2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

