Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits
- PMID: 19560048
- PMCID: PMC2742626
- DOI: 10.1016/j.semcdb.2009.01.007
Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits
Abstract
Nicotine is the principle addictive agent delivered via cigarette smoking. The addictive activity of nicotine is due to potent interactions with nicotinic acetylcholine receptors (nAChRs) on neurons in the reinforcement and reward circuits of the brain. Beyond its addictive actions, nicotine is thought to have positive effects on performance in working memory and short-term attention-related tasks. The brain areas involved in such behaviors are part of an extensive cortico-limbic network that includes relays between prefrontal cortex (PFC) and cingulate cortex (CC), hippocampus, amygdala, ventral tegmental area (VTA) and the nucleus accumbens (nAcc). Nicotine activates a broad array of nAChRs subtypes that can be targeted to pre- as well as peri- and post-synaptic locations in these areas. Thereby, nicotine not only excites different types of neurons, but it also perturbs baseline neuronal communication, alters synaptic properties and modulates synaptic plasticity. In this review we focus on recent findings on nicotinic modulation of cortical circuits and their targets fields, which show that acute and transient activation of nicotinic receptors in cortico-limbic circuits triggers a series of events that affects cognitive performance in a long lasting manner. Understanding how nicotine induces long-term changes in synapses and alters plasticity in the cortico-limbic circuits is essential to determining how these areas interact in decoding fundamental aspects of cognition and reward.
Figures

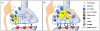
References
-
- Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53(4):447–56. - PubMed
-
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors halpha 2beta 2, halpha 2beta 4, halpha 3beta 2, halpha 3beta 4, halpha 4beta 2, halpha 4beta 4 and halpha 7 expressed in xenopus oocytes. J Pharmacol Exp Ther. 1997;280(1):346–56. - PubMed
-
- Changeux JP, Edelstein SJ. Allosteric mechanisms in normal and pathological nicotinic acetylcholine receptors. Curr Opin Neurobiol. 2001;11(3):369–77. - PubMed
-
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function, in reviews of physiology. Biochem Pharmacol. 2003;147:1–46. - PubMed
-
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74(6):363–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

