Mercury levels in premature and low birth weight newborn infants after receipt of thimerosal-containing vaccines
- PMID: 19560158
- PMCID: PMC2767297
- DOI: 10.1016/j.jpeds.2009.04.011
Mercury levels in premature and low birth weight newborn infants after receipt of thimerosal-containing vaccines
Abstract
Objective: We conducted a population-based pharmacokinetic study to assess blood levels and elimination of mercury after vaccination of premature infants born at > or =32 and <37 weeks of gestation and with birth weight > or =2000 but <3000 g.
Study design: Blood, stool, and urine samples were obtained before vaccination and 12 hours to 30 days after vaccination from 72 premature newborn infants. Total mercury levels were measured by atomic absorption.
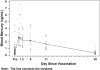
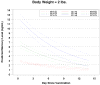
Results: The mean +/- standard deviation (SD) birth weight was 2.4 +/- 0.3 kg for the study population. Maximal mean +/- SD blood mercury level was 3.6 +/- 2.1 ng/mL, occurring at 1 day after vaccination; maximal mean +/- SD stool mercury level was 35.4 +/- 38.0 ng/g, occurring on day 5 after vaccination; and urine mercury levels were mostly nondetectable. The blood mercury half-life was calculated to be 6.3 (95% CI, 3.85 to 8.77) days, and mercury levels returned to prevaccination levels by day 30.
Conclusions: The blood half-life of intramuscular ethyl mercury from thimerosal in vaccines given to premature infants is substantially shorter than that of oral methyl mercury in adults. Because of the differing pharmacokinetics, exposure guidelines based on oral methyl mercury in adults may not be accurate for children who receive thimerosal-containing vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- American Academy of Pediatrics. Thimerosal in vaccines - An interim report to clinicians. Pediatrics. 1999;104:570–574. - PubMed
-
- Ball LK, Ball R, Pratt RD. An assessment of thimerosal use in childhood vaccines. Pediatrics. 2001;107:1147–1154. - PubMed
-
- US Environmental Protection Agency. Mercury study report to congress. US Environmental Protection Agency; 1997. EPA-452/R-97-007.
-
- Pichichero ME, Cernichiari E, Lopreiato J, Treanor J. Mercury concentrations and metabolism in infants receiving vaccines containing thimerosal: a descriptive study. Lancet. 2002;360:1737–1741. - PubMed
-
- Pichichero ME, Gentile A, Giglio N, Clarkson T, Zareba G. Mercury Levels in Newborns and Infants After Receipt of Thimerosal-Containing Vaccines. Pediatrics. 2008;121:208–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

