Modafinil for the treatment of cocaine dependence
- PMID: 19560290
- PMCID: PMC2818032
- DOI: 10.1016/j.drugalcdep.2009.04.015
Modafinil for the treatment of cocaine dependence
Abstract
Aim: Modafinil was tested for efficacy in facilitating abstinence in cocaine-dependent patients, compared to placebo.
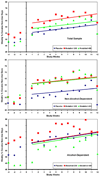
Methods: This was a double-blind placebo-controlled study, with 12 weeks of treatment and a 4-week follow-up. Six outpatient substance abuse treatment clinics participated in the study. There were 210 treatment-seekers randomized, having a diagnosis of cocaine dependence; 72 participants were randomized to placebo, 69 to modafinil 200mg, and 69 to modafinil 400mg, taken once daily on awakening. Participants came to the clinic three times per week for assessments and urine drug screens, and had one hour of individual psychotherapy weekly. The primary outcome measure was the weekly percentage of cocaine non-use days.
Results: The GEE regression analysis showed that for the total sample, there was no significant difference between either modafinil group and placebo in the change in average weekly percent of cocaine non-use days over the 12-week treatment period (p>0.79). However, two secondary outcomes showed significant effects by modafinil 200mg: the maximum number of consecutive non-use days for cocaine (p=0.02), and a reduction in craving (p=0.04). Also, a post hoc analysis showed a significant effect of modafinil that increased the weekly percentage of non-use days in the subgroup of those cocaine patients who did not have a history of alcohol dependence (p<0.02).
Conclusions: These data suggest that modafinil, in combination with individual behavioral therapy, was effective for increasing cocaine non-use days in participants without co-morbid alcohol dependence, and in reducing cocaine craving.
Conflict of interest statement
Charles Dackis has served as a consultant to Cephalon. Eugene Somoza has served as a consultant to Catalyst Pharmaceutical Partners. Domenic Ciraulo has a clinical trials contract with Catalyst Pharmaceuticals. All other authors declare that they have no conflicts of interest, i.e., no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted that could inappropriately influence, or be perceived to influence, this work.
Figures


References
-
- Cephalon, Inc. PROVIGIL (modafinil) Tablets [C-IV], FDA Approved Labeling dated August 17, 2007. Frazer, PA: Cephalon, Inc.; 2007.
-
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O’Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. - PubMed
-
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. - PubMed
-
- Donovan JL, DeVane CL, Malcolm RJ, Mojsiak J, Chiang CN, Elkashef A, Taylor RM. Modafinil influences the pharmacokinetics of intravenous cocaine in healthy cocaine-dependent volunteers. Clin. Pharmacokinet. 2005;44:753–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

