KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals
- PMID: 19560418
- PMCID: PMC2737517
- DOI: 10.1016/j.molcel.2009.06.001
KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals
Abstract
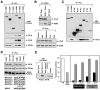
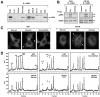
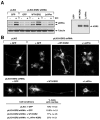
Protein scaffolds have emerged as important regulators of MAPK cascades, facilitating kinase activation and providing crucial spatio/temporal control to their signaling outputs. Using a proteomics approach to compare the binding partners of the two mammalian KSR scaffolds, we find that both KSR1 and KSR2 interact with the kinase components of the ERK cascade and have a common function in promoting RTK-mediated ERK signaling. Strikingly, we find that the protein phosphatase calcineurin selectively interacts with KSR2 and that KSR2 uniquely contributes to Ca2+-mediated ERK signaling. Calcineurin dephosphorylates KSR2 on specific sites in response to Ca2+ signals, thus regulating KSR2 localization and activity. Moreover, we find that depletion of endogenous KSR2 impairs Ca2+-mediated ERK activation and ERK-dependent signaling responses in INS1 pancreatic beta-cells and NG108 neuroblastoma cells. These findings identify KSR2 as a Ca2+-regulated ERK scaffold and reveal a new mechanism whereby Ca2+ impacts Ras to ERK pathway signaling.
Figures




Similar articles
-
Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK.Mol Cell Biol. 2012 Sep;32(18):3718-31. doi: 10.1128/MCB.06754-11. Epub 2012 Jul 16. Mol Cell Biol. 2012. PMID: 22801368 Free PMC article.
-
The KSR2-calcineurin complex regulates STIM1-ORAI1 dynamics and store-operated calcium entry (SOCE).Mol Biol Cell. 2014 Jun;25(11):1769-81. doi: 10.1091/mbc.E13-05-0292. Epub 2014 Mar 26. Mol Biol Cell. 2014. PMID: 24672054 Free PMC article.
-
A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK.Nature. 2011 Apr 21;472(7343):366-9. doi: 10.1038/nature09860. Epub 2011 Mar 27. Nature. 2011. PMID: 21441910
-
Coordinating ERK signaling via the molecular scaffold Kinase Suppressor of Ras.F1000Res. 2017 Aug 31;6:1621. doi: 10.12688/f1000research.11895.1. eCollection 2017. F1000Res. 2017. PMID: 29026529 Free PMC article. Review.
-
KSR as a therapeutic target for Ras-dependent cancers.Expert Opin Ther Targets. 2017 May;21(5):499-509. doi: 10.1080/14728222.2017.1311325. Epub 2017 Apr 7. Expert Opin Ther Targets. 2017. PMID: 28333549 Free PMC article. Review.
Cited by
-
Regulation of RAF protein kinases in ERK signalling.Nat Rev Mol Cell Biol. 2015 May;16(5):281-98. doi: 10.1038/nrm3979. Nat Rev Mol Cell Biol. 2015. PMID: 25907612 Review.
-
Signal control through Raf: in sickness and in health.Cell Res. 2012 Jan;22(1):14-22. doi: 10.1038/cr.2011.193. Epub 2011 Dec 6. Cell Res. 2012. PMID: 22143568 Free PMC article. Review.
-
Splicing factor SF3B1 promotes endometrial cancer progression via regulating KSR2 RNA maturation.Cell Death Dis. 2020 Oct 10;11(10):842. doi: 10.1038/s41419-020-03055-y. Cell Death Dis. 2020. PMID: 33040078 Free PMC article.
-
ERK Signals: Scaffolding Scaffolds?Front Cell Dev Biol. 2016 May 31;4:49. doi: 10.3389/fcell.2016.00049. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27303664 Free PMC article.
-
The optimized core peptide derived from CABIN1 efficiently inhibits calcineurin-mediated T-cell activation.Exp Mol Med. 2022 May;54(5):613-625. doi: 10.1038/s12276-022-00772-6. Epub 2022 May 12. Exp Mol Med. 2022. PMID: 35550603 Free PMC article.
References
-
- Arnette D, Gibson TB, Lawrence MC, January B, Khoo S, McGlynn K, Vanderbilt CA, Cobb MH. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem. 2003;278:32517–32525. - PubMed
-
- Cacace AM, Michaud NR, Therrien M, Mathes K, Copeland T, Rubin GM, Morrison DK. Identification of constitutive and ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol Cell Biol. 1999;19:229–240. - PMC - PubMed
-
- Channavajhala PL, Rao VR, Spaulding V, Lin LL, Zhang YG. hKSR-2 inhibits MEKK3-activated MAP kinase and NF-kappaB pathways in inflammation. Biochem Biophys Res Commun. 2005;334:1214–1218. - PubMed
-
- Channavajhala PL, Wu L, Cuozzo JW, Hall JP, Liu W, Lin LL, Zhang Y. Identification of a novel human kinase supporter of Ras (hKSR-2) that functions as a negative regulator of Cot (Tpl2) signaling. J Biol Chem. 2003;278:47089–47097. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

