Multiplex cytokine profiling with highly pathogenic material: use of formalin solution in luminex analysis
- PMID: 19560467
- PMCID: PMC7094240
- DOI: 10.1016/j.jim.2009.06.007
Multiplex cytokine profiling with highly pathogenic material: use of formalin solution in luminex analysis
Abstract
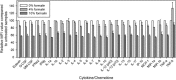
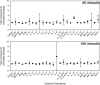
Work with highly pathogenic material mandates the use of biological containment facilities, involving microbiological safety cabinets and specialist laboratory engineering structures typified by containment level 3 (CL3) and CL4 laboratories. Consequences of working in high containment are the practical difficulties associated with containing specialist assays and equipment often essential for experimental analyses. In an era of increased interest in biodefence pathogens and emerging diseases, immunological analysis has developed rapidly alongside traditional techniques in virology and molecular biology. For example, in order to maximise the use of small sample volumes, multiplexing has become a more popular and widespread approach to quantify multiple analytes simultaneously, such as cytokines and chemokines. The luminex microsphere system allows for the detection of many cytokines and chemokines in a single sample, but the detection method of using aligned lasers and fluidics means that samples often have to be analysed in low containment facilities. In order to perform cytokine analysis in materials from high containment (CL3 and CL4 laboratories), we have developed an appropriate inactivation methodology after staining steps, which although results in a reduction of median fluorescent intensity, produces statistically comparable outcomes when judged against non-inactivated samples. This methodology thus extends the use of luminex technology for material that contains highly pathogenic biological agents.
Figures

Similar articles
-
Use and reliability of multiplex bead-based assays (Luminex) at Containment Level 4.Methods. 2019 Apr 1;158:17-21. doi: 10.1016/j.ymeth.2019.02.008. Epub 2019 Feb 13. Methods. 2019. PMID: 30771491
-
Efficient inactivation of Burkholderia pseudomallei or Francisella tularensis in infected cells for safe removal from biosafety level 3 containment laboratories.Pathog Dis. 2014 Jul;71(2):276-81. doi: 10.1111/2049-632X.12138. Epub 2014 Feb 18. Pathog Dis. 2014. PMID: 24449562 Free PMC article.
-
Evaluation of virus inactivation by formaldehyde to enhance biosafety of diagnostic electron microscopy.Viruses. 2015 Feb 10;7(2):666-79. doi: 10.3390/v7020666. Viruses. 2015. PMID: 25674771 Free PMC article.
-
Selection and application of biological safety cabinets in diagnostic and research laboratories with special emphasis on COVID-19.Rev Sci Instrum. 2021 Aug 1;92(8):081401. doi: 10.1063/5.0047716. Rev Sci Instrum. 2021. PMID: 34470433 Free PMC article. Review.
-
Evidence-based biosafety: a review of the principles and effectiveness of microbiological containment measures.Clin Microbiol Rev. 2008 Jul;21(3):403-25. doi: 10.1128/CMR.00014-08. Clin Microbiol Rev. 2008. PMID: 18625678 Free PMC article. Review.
Cited by
-
Comparison of Chikungunya Virus-Induced Disease Progression and Pathogenesis in Type-I Interferon Receptor-Deficient Mice (A129) and Two Wild-Type (129Sv/Ev and C57BL/6) Mouse Strains.Viruses. 2024 Sep 27;16(10):1534. doi: 10.3390/v16101534. Viruses. 2024. PMID: 39459867 Free PMC article.
-
A human-ACE2 knock-in mouse model for SARS-CoV-2 infection recapitulates respiratory disorders but avoids neurological disease associated with the transgenic K18-hACE2 model.mBio. 2025 May 14;16(5):e0072025. doi: 10.1128/mbio.00720-25. Epub 2025 Apr 24. mBio. 2025. PMID: 40272151 Free PMC article.
-
Pathogenesis of Rift Valley Fever Virus in a BALB/c Mouse Model Is Affected by Virus Culture Conditions and Sex of the Animals.Viruses. 2023 Nov 30;15(12):2369. doi: 10.3390/v15122369. Viruses. 2023. PMID: 38140610 Free PMC article.
References
-
- Aloisio C.H., Nicholson J.K. Recovery of infectious human immunodeficiency virus from cells treated with 1% paraformaldehyde. J. Immunol. Methods. 1990;128:281. - PubMed
-
- Bray M. Pathogenesis of viral hemorrhagic fever. Curr. Opin. Immunol. 2005;17:399. - PubMed
-
- Brown F. Inactivation of viruses by aziridines. Vaccine. 2001;20:322. - PubMed
-
- Canete M., Juarranz A., Lopez-Nieva P., Alonso-Torcal C., Villanueva A., Stockert J.C. Fixation and permanent mounting of fluorescent probes after vital labelling of cultured cells. Acta Histochem. 2001;103:117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

