DNA-tethered membranes formed by giant vesicle rupture
- PMID: 19560541
- PMCID: PMC2757119
- DOI: 10.1016/j.jsb.2009.06.015
DNA-tethered membranes formed by giant vesicle rupture
Abstract
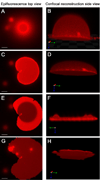
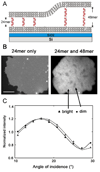
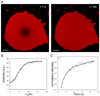
We have developed a strategy for preparing tethered lipid bilayer membrane patches on solid surfaces by DNA hybridization. In this way, the tethered membrane patch is held at a controllable distance from the surface by varying the length of the DNA used. Two basic strategies are described. In the first, single-stranded DNA strands are immobilized by click chemistry to a silica surface, whose remaining surface is passivated to prevent direct assembly of a solid supported bilayer. Then giant unilamellar vesicles (GUVs) displaying the antisense strand, using a DNA-lipid conjugate developed in earlier work [Chan, Y.-H.M., van Lengerich, B., et al., 2008. Lipid-anchored DNA mediates vesicle fusion as observed by lipid and content mixing. Biointerphases 3 (2), FA17-FA21], are allowed to tether, spread and rupture to form tethered bilayer patches. In the second, a supported lipid bilayer displaying DNA using the DNA-lipid conjugate is first assembled on the surface. Then GUVs displaying the antisense strand are allowed to tether, spread and rupture to form tethered bilayer patches. The essential difference between these methods is that the tethering hybrid DNA is immobile in the first, while it is mobile in the second. Both strategies are successful; however, with mobile DNA hybrids as tethers, the patches are unstable, while in the first strategy stable patches can be formed. In the case of mobile tethers, if different length DNA hybrids are present, lateral segregation by length occurs and can be visualized by fluorescence interference contrast microscopy making this an interesting model for interactions that occur in cell junctions. In both cases, lipid mobility is high and there is a negligible immobile fraction. Thus, these architectures offer a flexible platform for the assembly of lipid bilayers at a well-defined distance from a solid support.
Figures



References
-
- Ajo-Franklin CM, Yoshina-Ishii C, et al. Probing the structure of supported membranes and tethered oligonucleotides by fluorescence interference contrast microscopy. Langmuir. 2005;21(11):4976–4983. - PubMed
-
- Ali MB, Bessueille F, et al. Use of ultra-thin organic silane films for the improvement of gold adhesion to the silicon dioxide wafers for (bio)sensor applications. Materials Science and Engineering C. 2008;28:628–632.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

