Animal models of asthma
- PMID: 19561139
- PMCID: PMC2739768
- DOI: 10.1152/ajplung.00027.2009
Animal models of asthma
Abstract
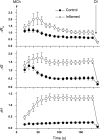
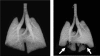
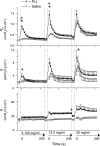
Studies in animal models form the basis for much of our current understanding of the pathophysiology of asthma, and are central to the preclinical development of drug therapies. No animal model completely recapitulates all features of the human disease, however. Research has focused primarily on ways to generate allergic inflammation by sensitizing and challenging animals with a variety of foreign proteins, leading to an increased understanding of the immunological factors that mediate the inflammatory response and its physiological expression in the form of airways hyperresponsiveness. Animal models of exaggerated airway narrowing are also lending support to the notion that asthma may represent an abnormality of the airway smooth muscle. The mouse is now the species of choice for asthma research involving animals. This presents practical challenges for physiological study because the mouse is so small, but modern imaging methodologies, coupled with the forced oscillation technique for measuring lung mechanics, have allowed the asthma phenotype in mice to be precisely characterized.
Figures

References
-
- Expert Panel Report 3 (EPR-3). Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. J Allergy Clin Immunol 120: S94–S138, 2007. - PubMed
-
- Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol 97: 286–292, 2004. - PubMed
-
- Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Med 9: 582–588, 2003. - PubMed
-
- Allen G, Bates JH. Dynamic mechanical consequences of deep inflation in mice depend on type and degree of lung injury. J Appl Physiol 96: 293–300, 2004. - PubMed
-
- Allen JE, Bischof RJ, Sucie Chang HY, Hirota JA, Hirst SJ, Inman MD, Mitzner W, Sutherland TE. Animal models of airway inflammation and airway smooth muscle remodelling in asthma. Pulm Pharmacol Ther. In press. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

