Comparative methylation of ERVWE1/syncytin-1 and other human endogenous retrovirus LTRs in placenta tissues
- PMID: 19561344
- PMCID: PMC2725788
- DOI: 10.1093/dnares/dsp011
Comparative methylation of ERVWE1/syncytin-1 and other human endogenous retrovirus LTRs in placenta tissues
Abstract
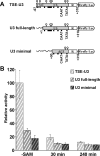
Human endogenous retroviruses (HERVs) are globally silent in somatic cells. However, some HERVs display high transcription in physiological conditions. In particular, ERVWE1, ERVFRDE1 and ERV3, three proviruses of distinct families, are highly transcribed in placenta and produce envelope proteins associated with placenta development. As silencing of repeated elements is thought to occur mainly by DNA methylation, we compared the methylation of ERVWE1 and related HERVs to appreciate whether HERV methylation relies upon the family, the integration site, the tissue, the long terminal repeat (LTR) function or the associated gene function. CpG methylation of HERV-W LTRs in placenta-associated tissues was heterogeneous but a joint epigenetic control was found for ERVWE1 5'LTR and its juxtaposed enhancer, a mammalian apparent LTR retrotransposon. Additionally, ERVWE1, ERVFRDE1 and ERV3 5'LTRs were all essentially hypomethylated in cytotrophoblasts during pregnancy, but showed distinct and stage-dependent methylation profiles. In non-cytotrophoblastic cells, they also exhibited different methylation profiles, compatible with their respective transcriptional activities. Comparative analyses of transcriptional activity and LTR methylation in cell lines further sustained a role for methylation in the control of functional LTRs. These results suggest that HERV methylation might not be family related but copy-specific, and related to the LTR function and the tissue. In particular, ERVWE1 and ERV3 could be developmentally epigenetically regulated HERVs.
Figures




Similar articles
-
Transcriptional derepression of the ERVWE1 locus following influenza A virus infection.J Virol. 2014 Apr;88(8):4328-37. doi: 10.1128/JVI.03628-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478419 Free PMC article.
-
A retroviral promoter and a cellular enhancer define a bipartite element which controls env ERVWE1 placental expression.J Virol. 2004 Nov;78(22):12157-68. doi: 10.1128/JVI.78.22.12157-12168.2004. J Virol. 2004. PMID: 15507602 Free PMC article.
-
Expression and regulation of human endogenous retrovirus W elements.APMIS. 2016 Jan-Feb;124(1-2):52-66. doi: 10.1111/apm.12478. APMIS. 2016. PMID: 26818262 Review.
-
DNA hypomethylation and aberrant expression of the human endogenous retrovirus ERVWE1/syncytin-1 in seminomas.Retrovirology. 2017 Mar 17;14(1):20. doi: 10.1186/s12977-017-0342-9. Retrovirology. 2017. PMID: 28302141 Free PMC article.
-
The important biological roles of Syncytin-1 of human endogenous retrovirus W (HERV-W) and Syncytin-2 of HERV-FRD in the human placenta development.Mol Biol Rep. 2023 Sep;50(9):7901-7907. doi: 10.1007/s11033-023-08658-0. Epub 2023 Jul 8. Mol Biol Rep. 2023. PMID: 37421503 Review.
Cited by
-
Multiple sclerosis: are protective immune mechanisms compromised by a complex infectious background?Autoimmune Dis. 2010 Dec 20;2011:708750. doi: 10.4061/2011/708750. Autoimmune Dis. 2010. PMID: 21197482 Free PMC article.
-
Analysis of DNA methylation in human BK virus.Virus Genes. 2011 Oct;43(2):201-7. doi: 10.1007/s11262-011-0627-3. Epub 2011 May 31. Virus Genes. 2011. PMID: 21626299
-
Endogenous Retroviruses: With Us and against Us.Front Chem. 2017 Apr 7;5:23. doi: 10.3389/fchem.2017.00023. eCollection 2017. Front Chem. 2017. PMID: 28439515 Free PMC article. Review.
-
Endogenous Retrovirus 3 - History, Physiology, and Pathology.Front Microbiol. 2018 Jan 15;8:2691. doi: 10.3389/fmicb.2017.02691. eCollection 2017. Front Microbiol. 2018. PMID: 29379485 Free PMC article. Review.
-
HERV Envelope Proteins: Physiological Role and Pathogenic Potential in Cancer and Autoimmunity.Front Microbiol. 2018 Mar 14;9:462. doi: 10.3389/fmicb.2018.00462. eCollection 2018. Front Microbiol. 2018. PMID: 29593697 Free PMC article. Review.
References
-
- Mager D. L., Medstrand P. Retroviral Repeat Sequences, Encyclopedia of Life Sciences. Chichester: John Wiley & Sons, Ltd; 2005.
-
- Lander E. S., Linton L. M., Birren B., Nusbaum C., Zody M. C., Baldwin J., et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
-
- Prudhomme S., Bonnaud B., Mallet F. Endogenous retroviruses and animal reproduction. Cytogenet. Genome Res. 2005;110:353–364. - PubMed
-
- Leib-Mösch C., Seifarth W., Schön U. Influence of human endogenous retroviruses on cellular gene expression. In: Serdlov E., editor. Retroviruses and Primate Genome Evolution. Georgetown, Texas USA: Landes Bioscience; 2005. pp. 123–143.
-
- Gimenez J., Mallet F. ERVWE1 (Endogenous Retroviral family W, Env(C7), member 1) Atlas Genet. Cytogenet. Oncol. Haematol. 2007 http://AtlasGeneticsOncology.org/Genes/ERVWE1ID40497ch7q21.html .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

