Detection of drug-induced cellular changes using confocal Raman spectroscopy on patterned single-cell biosensors
- PMID: 19562213
- PMCID: PMC2902718
- DOI: 10.1039/b900420c
Detection of drug-induced cellular changes using confocal Raman spectroscopy on patterned single-cell biosensors
Abstract
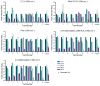
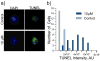
We report on a cell-based biosensor application that utilizes patterned single-cell arrays combined with confocal Raman spectroscopy to observe the time-dependent drug response of individual cells in real time. The patterned single-cell platform enables individual cells to be easily located and continuously addressable for Raman spectroscopy characterization of biochemical compositional changes in a non-destructive, quantitative manner so that discrete cellular behavior and cell-to-cell variations are preserved. In this study, human medulloblastoma (DAOY) cells were exposed to the common chemotherapeutic agent etoposide, and Raman spectra from patterned cells were recorded over 48 hours. It was found that 87.5% of the cells monitored exhibited a sharp decrease in DNA and protein associated peaks 48 hours after drug exposure, corresponding to cell death. The remaining 12.5% of the cells showed little to no reduction in key Raman biomarkers, indicating their drug resistance. Furthermore, the patterned cell population showed a very similar response to etoposide as confluent cell cultures, as confirmed by flow cytometry. Finally, patterned cells were assessed with TUNEL assay for apoptosis due to DNA fragmentation after etoposide exposure. The results agree well with those from the Raman spectroscopy analysis. This combined biosensor-Raman platform provides a quick, simple way to assess cell responses to chemical and biological agents with high throughput and can be potentially used for a wide variety of biomedical applications such as pharmaceutical drug discovery, toxin tests, and biothreat detection.
Figures



Similar articles
-
New detection system for toxic agents based on continuous spectroscopic monitoring of living cells.Biosens Bioelectron. 2004 Nov 1;20(4):780-9. doi: 10.1016/j.bios.2004.04.008. Biosens Bioelectron. 2004. PMID: 15522593
-
In vitro toxicology evaluation of pharmaceuticals using Raman micro-spectroscopy.J Cell Biochem. 2006 Sep 1;99(1):178-86. doi: 10.1002/jcb.20884. J Cell Biochem. 2006. PMID: 16598770
-
Single-cell bioelectrical impedance platform for monitoring cellular response to drug treatment.Phys Biol. 2011 Feb;8(1):015006. doi: 10.1088/1478-3975/8/1/015006. Epub 2011 Feb 7. Phys Biol. 2011. PMID: 21301069 Free PMC article.
-
The Recent Advances in Raman Microscopy and Imaging Techniques for Biosensors.Biosensors (Basel). 2019 Feb 12;9(1):25. doi: 10.3390/bios9010025. Biosensors (Basel). 2019. PMID: 30759840 Free PMC article. Review.
-
High-Throughput Raman Flow Cytometry and Beyond.Acc Chem Res. 2021 May 4;54(9):2132-2143. doi: 10.1021/acs.accounts.1c00001. Epub 2021 Mar 31. Acc Chem Res. 2021. PMID: 33788539 Review.
Cited by
-
Metabolic toxicity screening using electrochemiluminescence arrays coupled with enzyme-DNA biocolloid reactors and liquid chromatography-mass spectrometry.Annu Rev Anal Chem (Palo Alto Calif). 2012;5(1):79-105. doi: 10.1146/annurev.anchem.111808.073659. Epub 2012 Apr 5. Annu Rev Anal Chem (Palo Alto Calif). 2012. PMID: 22482786 Free PMC article. Review.
-
Raman spectrum spectral imaging revealing the molecular mechanism of Berberine-induced Jurkat cell apoptosis and the receptor-mediated Berberine delivery system.Biomed Opt Express. 2019 Mar 4;10(4):1581-1600. doi: 10.1364/BOE.10.001581. eCollection 2019 Apr 1. Biomed Opt Express. 2019. PMID: 31061758 Free PMC article.
-
Single-cell Raman and mass spectrometry analysis to probe cellular heterogeneity in tamoxifen uptake and metabolism.Anal Bioanal Chem. 2025 Sep;417(23):5349-5358. doi: 10.1007/s00216-025-06058-w. Epub 2025 Aug 14. Anal Bioanal Chem. 2025. PMID: 40810750 Free PMC article.
-
Detection of doxorubicin-induced apoptosis of leukemic T-lymphocytes by laser tweezers Raman spectroscopy.Biomed Opt Express. 2010 Oct 10;1(4):1138-1147. doi: 10.1364/BOE.1.001138. Biomed Opt Express. 2010. PMID: 21258536 Free PMC article.
-
Visualizing cell state transition using Raman spectroscopy.PLoS One. 2014 Jan 7;9(1):e84478. doi: 10.1371/journal.pone.0084478. eCollection 2014. PLoS One. 2014. PMID: 24409302 Free PMC article.
References
-
- Pancrazio JJ, Whelan JP, Borkholder DA, Ma W, Stenger DA. Annals of Biomedical Engineering. 1999;27:697–711. - PubMed
-
- Lorenzelli L, Margesin B, Martinoia S, Tedesco MT, Valle M. Biosensors & Bioelectronics. 2003;18:621–626. - PubMed
-
- Levsky JM, Singer RH. Trends in Cell Biology. 2003;13:4–6. - PubMed
-
- Chen CS, Jiang XY, Whitesides GM. Mrs Bulletin. 2005;30:194–201.
-
- Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dolle MET, Calder RB, Chisholm GB, Pollock BH, Klein CA, Vijg J. Nature. 2006;441:1011–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

