Continuous infusion of enzyme replacement therapy is inferior to weekly infusions in MPS I dogs
- PMID: 19562502
- PMCID: PMC2889197
- DOI: 10.1007/s10545-009-1198-5
Continuous infusion of enzyme replacement therapy is inferior to weekly infusions in MPS I dogs
Abstract
Intravenous enzyme replacement therapy with recombinant human α-L-iduronidase (rhIDU) is used weekly to treat mucopolysaccharidosis (MPS) I. We tested continuous administration of rhIDU at two dosing levels (0.58 mg/kg per week and 2 mg/kg per week) in MPS I dogs, and compared the efficacy of continuous infusion with the clinically used 0.58 mg/kg weekly three-hour infusion. Peak plasma concentrations of rhIDU were much higher in weekly-treated dogs (mean 256 units/ml) than steady-state concentrations in dogs treated with continuous infusion (mean 1.97 units/ml at 0.58 mg/kg per week; 8.44 units/ml at 2 mg/kg per week). Dogs receiving continuous IV rhIDU, even at a higher (2 mg/kg per week) dose, had consistently lower iduronidase levels in tissues than dogs receiving a weekly (0.58 mg/kg per week) dose. GAG storage was also less improved by continuous intravenous infusion. Adverse events were similar in all dosing groups. We found that continuous administration of 2 mg/kg per week rhIDU to MPS I dogs was insufficient to achieve GAG storage reduction comparable to 0.58 mg/kg weekly dosing.
Figures
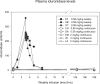

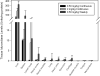
References
-
- Barton NW, Brady RO, Dambrosia JM, et al. Replacement therapy for inherited enzyme deficiency: macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991;324:1464–1470. - PubMed
-
- Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human a-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. - PubMed
-
- Giugliani R, Rojas VM, Martins AM, et al. A dose-optimization trial of laronidase (Aldurazyme) in patients with mucopolysaccharidosis I. Mol Genet Metab. 2009;96:13–19. - PubMed
-
- Harmatz P, Kramer WG, Hopwood JJ, et al. Pharmacokinetic profile of recombinant human N-acetylgalactosamine 4-sulphatase enzyme replacement therapy in patients with mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): a phase I/II study. Acta Pædiatr Suppl. 2005;94:61–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

