Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia
- PMID: 19563516
- PMCID: PMC2757454
- DOI: 10.1111/j.1601-183X.2009.00504.x
Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia
Abstract
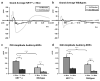
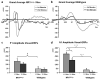
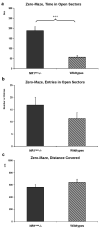
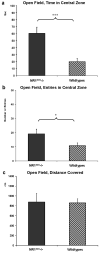
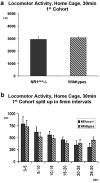
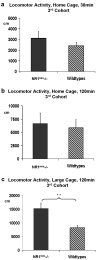
N-methyl-D-aspartate receptors (NMDARs) play a pivotal role in excitatory neurotransmission, synaptic plasticity and brain development. Clinical and experimental evidence suggests a dysregulation of NMDAR function and glutamatergic pathways in the pathophysiology of schizophrenia. We evaluated electrophysiological and behavioral properties of NMDAR deficiency utilizing mice that express only 5-10% of the normal level of NMDAR NR1 subunit. Auditory and visual event related potentials yielded significantly increased amplitudes for the P20 and N40 components in NMDAR deficient (NR1(neo)-/-) mice suggesting decreased inhibitory tone. Compared to wild types, NR1(neo)-/- mice spent less time in social interactions and showed reduced nest building. NR1(neo)-/- mice displayed a preference for open arms of a zero maze and central zone of an open field, possibly reflecting decreased anxiety-related behavioral inhibition. However, locomotor activity did not differ between groups in either home cage environment or during behavioral testing. NR1(neo)-/- mice displayed hyperactivity only when placed in a large unfamiliar environment, suggesting that neither increased anxiety nor non-specific motor activation accounts for differential behavioral patterns. Data suggest that NMDAR NR1 deficiency causes disinhibition in sensory processing as well as reduced behavioral inhibition and impaired social interactions. The behavioral signature in NR1(neo)-/- mice supports the impact of impaired NMDAR function in a mouse model with possible relevance to negative symptoms in schizophrenia.
Figures


References
-
- Ballard TM, Pauly-Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, Kemp JA, Bluethmann H, Kew JN. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neurosci. 2002;22:6713–6723. - PMC - PubMed
-
- Bickel S, Lipp HP, Umbricht D. Early Auditory Sensory Processing Deficits in Mouse Mutants with Reduced NMDA Receptor Function. Neuropsychopharmacology. 2008;33:1680–1689. - PubMed
-
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behavioural brain research. 2007;176:53–65. - PubMed
-
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain research. 2004;1002:151–157. - PubMed
-
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

