A new class of organic nitrates: investigations on bioactivation, tolerance and cross-tolerance phenomena
- PMID: 19563531
- PMCID: PMC2757691
- DOI: 10.1111/j.1476-5381.2009.00303.x
A new class of organic nitrates: investigations on bioactivation, tolerance and cross-tolerance phenomena
Erratum in
- Br J Pharmacol. 2013 Jun;169(4):952
Abstract
Background and purpose: The chronic use of organic nitrates is limited by serious side effects including oxidative stress, nitrate tolerance and/or endothelial dysfunction. The side effects and potency of nitroglycerine depend on mitochondrial aldehyde dehydrogenase (ALDH-2). We sought to determine whether this concept can be extended to a new class of organic nitrates with amino moieties (aminoalkyl nitrates).
Experimental approach: Vasodilator potency of the organic nitrates, in vitro tolerance and in vivo tolerance (after continuous infusion for 3 days) were assessed in wild-type and ALDH-2 knockout mice by isometric tension studies. Mitochondrial oxidative stress was analysed by L-012-dependent chemiluminescence and protein tyrosine nitration.
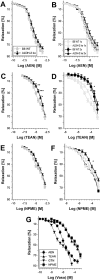
Key results: Aminoethyl nitrate (AEN) showed an almost similar potency to glyceryl trinitrate (GTN), even though it is only a mononitrate. AEN-dependent vasodilatation was mediated by cGMP and nitric oxide. In contrast to triethanolamine trinitrate (TEAN) and GTN, AEN bioactivation did not depend on ALDH-2 and caused no in vitro tolerance. In vivo treatment with TEAN and GTN, but not with AEN, induced cross-tolerance to acetylcholine (ACh)-dependent and GTN-dependent relaxation. Although all nitrates tested induced tolerance to themselves, only TEAN and GTN significantly increased mitochondrial oxidative stress in vitro and in vivo.
Conclusions and implications: The present results demonstrate that not all high potency nitrates are bioactivated by ALDH-2 and that high potency of a given nitrate is not necessarily associated with induction of oxidative stress or nitrate tolerance. Obviously, there are distinct pathways for bioactivation of organic nitrates, which for AEN may involve xanthine oxidoreductase rather than P450 enzymes.
Figures





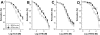
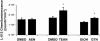
Comment in
-
Aminoethyl nitrate--the novel super nitrate?Br J Pharmacol. 2009 Sep;158(2):507-9. doi: 10.1111/j.1476-5381.2009.00414.x. Br J Pharmacol. 2009. PMID: 19732062 Free PMC article.
-
2-Aminoethylnitrate: Earlier investigation as a drug was missed by recent authors due to changes in nomenclature.Br J Pharmacol. 2013 Jun;169(4):949-50. doi: 10.1111/bph.12148. Br J Pharmacol. 2013. PMID: 23711023 Free PMC article. No abstract available.
-
2-Aminoethylnitrate: pharmacological uses rediscovered and claimed as original.Br J Pharmacol. 2013 Jun;169(4):950. doi: 10.1111/bph.12146. Br J Pharmacol. 2013. PMID: 23711024 Free PMC article. No abstract available.
-
Response to a letter to the editor by Satvika Uppu.Br J Pharmacol. 2013 Jun;169(4):951-2. doi: 10.1111/bph.12147. Br J Pharmacol. 2013. PMID: 23711025 Free PMC article. No abstract available.
References
-
- Abrams J. Beneficial actions of nitrates in cardiovascular disease. Am J Cardiol. 1996;77(13):31C–37C. - PubMed
-
- Anderson TJ, Meredith IT, Ganz P, Selwyn AP, Yeung AC. Nitric oxide and nitrovasodilators: similarities, differences and potential interactions. J Am Coll Cardiol. 1994;24(2):555–566. - PubMed
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

