Sphingolipids in inflammation: pathological implications and potential therapeutic targets
- PMID: 19563535
- PMCID: PMC2785521
- DOI: 10.1111/j.1476-5381.2009.00281.x
Sphingolipids in inflammation: pathological implications and potential therapeutic targets
Abstract
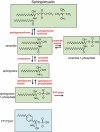
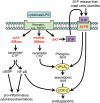
Sphingolipids are formed via the metabolism of sphingomyelin, a constituent of the plasma membrane, or by de novo synthesis. Enzymatic pathways result in the formation of several different lipid mediators, which are known to have important roles in many cellular processes, including proliferation, apoptosis and migration. Several studies now suggest that these sphingolipid mediators, including ceramide, ceramide 1-phosphate and sphingosine 1-phosphate (S1P), are likely to have an integral role in inflammation. This can involve, for example, activation of pro-inflammatory transcription factors in different cell types and induction of cyclooxygenase-2, leading to production of pro-inflammatory prostaglandins. The mode of action of each sphingolipid is different. Increased ceramide production leads to the formation of ceramide-rich areas of the membrane, which may assemble signalling complexes, whereas S1P acts via high-affinity G-protein-coupled S1P receptors on the plasma membrane. Recent studies have demonstrated that in vitro effects of sphingolipids on inflammation can translate into in vivo models. This review will highlight the areas of research where sphingolipids are involved in inflammation and the mechanisms of action of each mediator. In addition, the therapeutic potential of drugs that alter sphingolipid actions will be examined with reference to disease states, such as asthma and inflammatory bowel disease, which involve important inflammatory components. A significant body of research now indicates that sphingolipids are intimately involved in the inflammatory process and recent studies have demonstrated that these lipids, together with associated enzymes and receptors, can provide effective drug targets for the treatment of pathological inflammation.
Figures
References
-
- Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–428. - PubMed
-
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15:1212–1214. - PubMed
-
- Anderson GP, Coyle AJ. Th2 and Th2 like cells in allergy and asthma: pharmacological perspectives. Trends Pharmacol Sci. 1994;15:324–332. - PubMed
-
- Billich A, Bornancin F, De'vay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. - PubMed
-
- Braegger CP, MacDonald TT. Immune mechanisms in chronic inflammatory bowel disease. Ann Allergy. 1994;72:135–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

