Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer
- PMID: 19563669
- PMCID: PMC2716510
- DOI: 10.1186/bcr2328
Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer
Abstract
Introduction: Inhibitor of apoptosis (IAPs) proteins are a family of proteins that can block apoptosis in normal cells and have been suggested to cause resistance to apoptosis in cancer. Overexpression of oncogenic receptor tyrosine kinases is common in breast cancer; in particular 20% of all cases show elevated Her2. Despite clinical success with the use of targeted therapies, such as Trastuzumab, only up to 35% of Her2-positive patients initially respond. We reasoned that IAP-mediated apoptosis resistance might contribute to this insensitivity to receptor tyrosine kinase therapy, in particular ErbB antagonists. Here we examine the levels of IAPs in breast cancer and evaluate whether targeting IAPs can enhance apoptosis in response to growth factor receptor antagonists and TRAIL.
Methods: IAP levels were examined in a breast cancer cell line panel and in patient samples. IAPs were inhibited using siRNA or cell permeable mimetics of endogenous inhibitors. Cells were then exposed to TRAIL, Trastuzumab, Lapatinib, or Gefitinib for 48 hours. Examining nuclear morphology and staining for cleaved caspase 3 was used to score apoptosis. Proliferation was examined by Ki67 staining.
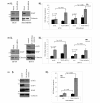
Results: Four members of the IAP family, Survivin, XIAP, cIAP1 and cIAP2, were all expressed to varying extents in breast cancer cell lines or tumours. MDAMB468, BT474 and BT20 cells all expressed XIAP to varying extents. Depleting the cells of XIAP overcame the intrinsic resistance of BT20 and MDAMB468 cells to TRAIL. Moreover, siRNA-based depletion of XIAP or use of a Smac mimetic to target multiple IAPs increased apoptosis in response to the ErbB antagonists, Trastuzumab, Lapatinib or Gefitinib in Her2-overexpressing BT474 cells, or Gefitinib in EGFR-overexpressing MDAMB468 cells.
Conclusions: The novel findings of this study are that multiple IAPs are concomitantly expressed in breast cancers, and that, in combination with clinically relevant Her2 treatments, IAP antagonists promote apoptosis and reduce the cell turnover index of breast cancers. We also show that combination therapy of IAP antagonists with some pro-apoptotic agents (for example, TRAIL) enhances apoptosis of breast cancer cells. In some cases (for example, MDAMB468 cells), the enhanced apoptosis is profound.
Figures


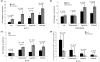

Similar articles
-
Smac mimetic reverses resistance to TRAIL and chemotherapy in human urothelial cancer cells.Cancer Biol Ther. 2010 Nov 1;10(9):885-92. doi: 10.4161/cbt.10.9.13237. Epub 2010 Nov 1. Cancer Biol Ther. 2010. PMID: 20814238 Free PMC article.
-
A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells.Oncogene. 2005 Nov 10;24(49):7381-8. doi: 10.1038/sj.onc.1208888. Oncogene. 2005. PMID: 16044155
-
X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO).Mol Cancer Ther. 2002 Oct;1(12):1051-8. Mol Cancer Ther. 2002. PMID: 12481428
-
Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs).Apoptosis. 2017 Jul;22(7):898-919. doi: 10.1007/s10495-017-1375-1. Apoptosis. 2017. PMID: 28424988 Free PMC article. Review.
-
Inhibitors of apoptosis proteins (IAPs) as potential molecular targets for therapy of hematological malignancies.Curr Mol Med. 2011 Nov;11(8):633-49. doi: 10.2174/156652411797536723. Curr Mol Med. 2011. PMID: 21902653 Review.
Cited by
-
Potential Diagnostic, Prognostic and Therapeutic Targets of MicroRNAs in Human Gastric Cancer.Int J Mol Sci. 2016 Jun 16;17(6):945. doi: 10.3390/ijms17060945. Int J Mol Sci. 2016. PMID: 27322246 Free PMC article. Review.
-
Smac mimetic compounds potentiate interleukin-1beta-mediated cell death.J Biol Chem. 2010 Dec 24;285(52):40612-23. doi: 10.1074/jbc.M110.183616. Epub 2010 Oct 18. J Biol Chem. 2010. PMID: 20956527 Free PMC article.
-
Inhibitor of Apoptosis Proteins: Promising Targets for Cancer Therapy.J Carcinog Mutagen. 2013 May 27;Suppl 14:S14-004. doi: 10.4172/2157-2518.S14-004. J Carcinog Mutagen. 2013. PMID: 25328816 Free PMC article.
-
Effects of polygonum aviculare herbal extract on proliferation and apoptotic gene expression of MCF-7.Daru. 2011;19(5):326-31. Daru. 2011. PMID: 22615677 Free PMC article.
-
Regulation of Apoptosis by HER2 in Breast Cancer.J Carcinog Mutagen. 2013;2013(Suppl 7):003. doi: 10.4172/2157-2518.S7-003. Epub 2013 Jun 26. J Carcinog Mutagen. 2013. PMID: 27088047 Free PMC article.
References
-
- Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815–6824. - PubMed
-
- Morizane Y, Honda R, Fukami K, Yasuda H. X-linked inhibitor of apoptosis functions as ubiquitin ligase toward mature caspase-9 and cytosolic Smac/DIABLO. J Biochem (Tokyo) 2005;137:125–132. - PubMed
-
- Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

