Comparison of metal-dependent catalysis by HIV-1 and ASV integrase proteins using a new and rapid, moderate throughput assay for joining activity in solution
- PMID: 19563676
- PMCID: PMC2717984
- DOI: 10.1186/1742-6405-6-14
Comparison of metal-dependent catalysis by HIV-1 and ASV integrase proteins using a new and rapid, moderate throughput assay for joining activity in solution
Abstract
Background: HIV-1 integrase (IN) is an attractive target for the development of drugs to treat AIDS, and inhibitors of this viral enzyme are already in the clinic. Nevertheless, there is a continuing need to devise new approaches to block the activity of this viral protein because of the emergence of resistant strains. To facilitate the biochemical analysis of wild-type IN and its derivatives, and to measure the potency of prospective inhibitory compounds, a rapid, moderate throughput solution assay was developed for IN-catalyzed joining of viral and target DNAs, based on the detection of a fluorescent tag.
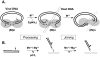
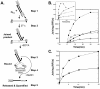
Results: A detailed, step-by-step description of the new joining assay is provided. The reactions are run in solution, the products captured on streptavidin beads, and activity is measured by release of a fluorescent tag. The procedure can be scaled up for the analysis of numerous samples, and is substantially more rapid and sensitive than the standard radioactive gel methods. The new assay is validated and its utility demonstrated via a detailed comparison of the Mg++- and Mn++-dependent activities of the IN proteins from human immunodeficiency virus type 1 (HIV-1) and the avian sarcoma virus (ASV). The results confirm that ASV IN is considerably more active than HIV-1 IN, but with both enzymes the initial rates of joining, and the product yields, are higher in the presence of Mn++ than Mg++. Although the pH optima for these two enzymes are similar with Mn++, they differ significantly in the presence of Mg++, which is likely due to differences in the molecular environment of the binding region of this physiologically relevant divalent cation. This interpretation is strengthened by the observation that a compound that can inhibit HIV-1 IN in the presence of either metal cofactors is only effective against ASV in the presence of Mn++.
Conclusion: A simplified, assay for measuring the joining activity of retroviral IN in solution is described, which offers several advantages over previous methods and the standard radioactive gel analyses. Based on comparisons of signal to background ratios, the assay is 10-30 times more sensitive than gel analysis, allows more rapid and accurate biochemical analyses of IN catalytic activity, and moderate throughput screening of inhibitory compounds. The assay is validated, and its utility demonstrated in a comparison of the metal-dependent activities of HIV-1 and ASV IN proteins.
Figures


References
-
- AIDSinfo SIDA. A Service of the US Department of Health and Human Services. http://www.aidsinfo.nih.gov
-
- Coffin JM, Hughes SH, Varmus HE. The Retroviruses. Cold Spring Harbor Laboratory Press; 1997. - PubMed
-
- Flint SJ, Enquist LW, Racaniello VR, Skalka AM. Principles of Virology Molecular Biology, Pathogenesis and Control of Animal Viruses. 2. Washington, DC: ASM Press; 2004.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

