PKCepsilon-dependent potentiation of TTX-resistant Nav1.8 current by neurokinin-1 receptor activation in rat dorsal root ganglion neurons
- PMID: 19563686
- PMCID: PMC2715383
- DOI: 10.1186/1744-8069-5-33
PKCepsilon-dependent potentiation of TTX-resistant Nav1.8 current by neurokinin-1 receptor activation in rat dorsal root ganglion neurons
Abstract
Background: Substance P (SP), which mainly exists in a subtype of small-diameter dorsal root ganglion (DRG) neurons, is an important signal molecule in pain processing in the spinal cord. Our previous results have proved the expression of SP receptor neurokinin-1 (NK-1) on DRG neurons and its interaction with transient receptor potential vanilloid 1 (TRPV1) receptor.
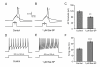
Results: In this study we investigated the effect of NK-1 receptor agonist on Na(v)1.8, a tetrodotoxin (TTX)-resistant sodium channel, in rat small-diameter DRG neurons employing whole-cell patch clamp recordings. NK-1 agonist [Sar(9), Met(O2)(11)]-substance P (Sar-SP) significantly enhanced the Na(v)1.8 currents in a subgroup of small-diameter DRG neurons under both the normal and inflammatory situation, and the enhancement was blocked by NK-1 antagonist Win51708 and protein kinase C (PKC) inhibitor bisindolylmaleimide (BIM), but not the protein kinase A (PKA) inhibitor H89. In particular, the inhibitor of PKCepsilon, a PKC isoform, completely blocked this effect. Under current clamp model, Sar-SP reduced the amount of current required to evoke action potentials and increased the firing rate in a subgroup of DRG neurons.
Conclusion: These data suggest that activation of NK-1 receptor potentiates Na(v)1.8 sodium current via PKCepsilon-dependent signaling pathway, probably participating in the generation of inflammatory hyperalgesia.
Figures




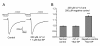
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

