Molecular mechanisms of endothelial hyperpermeability: implications in inflammation
- PMID: 19563700
- PMCID: PMC2828491
- DOI: 10.1017/S1462399409001112
Molecular mechanisms of endothelial hyperpermeability: implications in inflammation
Abstract
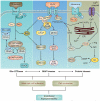
Endothelial hyperpermeability is a significant problem in vascular inflammation associated with trauma, ischaemia-reperfusion injury, sepsis, adult respiratory distress syndrome, diabetes, thrombosis and cancer. An important mechanism underlying this process is increased paracellular leakage of plasma fluid and protein. Inflammatory stimuli such as histamine, thrombin, vascular endothelial growth factor and activated neutrophils can cause dissociation of cell-cell junctions between endothelial cells as well as cytoskeleton contraction, leading to a widened intercellular space that facilitates transendothelial flux. Such structural changes initiate with agonist-receptor binding, followed by activation of intracellular signalling molecules including calcium, protein kinase C, tyrosine kinases, myosin light chain kinase, and small Rho-GTPases; these kinases and GTPases then phosphorylate or alter the conformation of different subcellular components that control cell-cell adhesion, resulting in paracellular hypermeability. Targeting key signalling molecules that mediate endothelial-junction-cytoskeleton dissociation demonstrates a therapeutic potential to improve vascular barrier function during inflammatory injury.
Figures

References
-
- Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circulation Research. 2004;94:1408–1417. - PubMed
-
- Lin MI, et al. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Research. 2007;67:2849–2856. - PubMed
-
- Razani B, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. Journal of Biological Chemistry. 2001;276:38121–38138. - PubMed
-
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiological Reviews. 2006;86:279–367. - PubMed
Further reading, resources and contacts
-
- Mammoto A, et al. Rho signaling and mechanical control of vascular development. Vascular Biology. 2008;15:228–234. This is an in-depth review on the impact of mechanical forces on endothelial barrier function. - PubMed
-
- van Nieuw Amerongen GP, et al. Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vascular Pharmacology. 2003;39:257–272. This is an extensive and detailed review of different strategies used to target endothelial barrier dysfunction. - PubMed
-
- Groeneveld AB, et al. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vascular Pharmacology. 2003;39:247–256. This article reviews the pathogenesis and current therapeutic strategies for adult respiratory distress syndrome. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

