Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation
- PMID: 19563755
- PMCID: PMC2729323
- DOI: 10.1016/j.cell.2009.04.022
Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation
Abstract
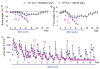
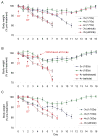
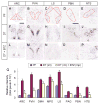
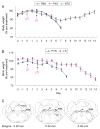
Neurons in the arcuate nucleus that produce AgRP, NPY, and GABA (AgRP neurons) promote feeding. Ablation of AgRP neurons in adult mice results in Fos activation in postsynaptic neurons and starvation. Loss of GABA is implicated in starvation because chronic subcutaneous delivery of bretazenil (a GABA(A) receptor partial agonist) suppresses Fos activation and maintains feeding during ablation of AgRP neurons. Moreover, under these conditions, direct delivery of bretazenil into the parabrachial nucleus (PBN), a direct target of AgRP neurons that also relays gustatory and visceral sensory information, is sufficient to maintain feeding. Conversely, inactivation of GABA biosynthesis in the ARC or blockade of GABA(A) receptors in the PBN of mice promote anorexia. We suggest that activation of the PBN by AgRP neuron ablation or gastrointestinal malaise inhibits feeding. Chronic delivery of bretazenil during loss of AgRP neurons provides time to establish compensatory mechanisms that eventually allow mice to eat.
Figures

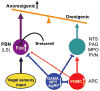
Comment in
-
GABA keeps up an appetite for life.Cell. 2009 Jun 26;137(7):1177-9. doi: 10.1016/j.cell.2009.06.002. Cell. 2009. PMID: 19563747
References
-
- Andre V, Dube C, Francois J, Leroy C, Rigoulot MA, Roch C, Namer IJ, Nehlig A. Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia. 2007;48 5:41–47. - PubMed
-
- Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev. 1996;20:189–287. - PubMed
-
- Berridge KC, Pecina S. Benzodiazepines, appetite, and taste palatability. Neurosci Biobehav Rev. 1995;19:121–131. - PubMed
-
- Broberger C, Hokfelt T. Hypothalamic and vagal neuropeptide circuitries regulating food intake. Physiol Behav. 2001;74:669–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

