Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut
- PMID: 19563763
- PMCID: PMC2753793
- DOI: 10.1016/j.cell.2009.05.014
Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut
Abstract
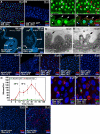
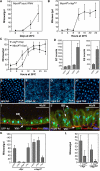
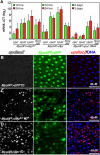
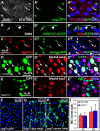
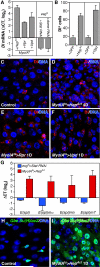
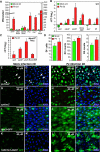
Cells in intestinal epithelia turn over rapidly due to damage from digestion and toxins produced by the enteric microbiota. Gut homeostasis is maintained by intestinal stem cells (ISCs) that divide to replenish the intestinal epithelium, but little is known about how ISC division and differentiation are coordinated with epithelial cell loss. We show here that when enterocytes (ECs) in the Drosophila midgut are subjected to apoptosis, enteric infection, or JNK-mediated stress signaling, they produce cytokines (Upd, Upd2, and Upd3) that activate Jak/Stat signaling in ISCs, promoting their rapid division. Upd/Jak/Stat activity also promotes progenitor cell differentiation, in part by stimulating Delta/Notch signaling, and is required for differentiation in both normal and regenerating midguts. Hence, cytokine-mediated feedback enables stem cells to replace spent progeny as they are lost, thereby establishing gut homeostasis.
Figures

Comment in
-
Fly stem cell research gets infectious.Cell. 2009 Jun 26;137(7):1185-7. doi: 10.1016/j.cell.2009.06.006. Cell. 2009. PMID: 19563751
References
-
- Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. - PMC - PubMed
-
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5:441–450. - PubMed
-
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. - PubMed
-
- Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

