Intrapartum antibiotic exposure and early neonatal, morbidity, and mortality in Africa
- PMID: 19564260
- PMCID: PMC2764263
- DOI: 10.1542/peds.2008-1873
Intrapartum antibiotic exposure and early neonatal, morbidity, and mortality in Africa
Abstract
Background: Infants born to women who receive intrapartum antibiotics may have higher rates of infectious morbidity and mortality than unexposed infants.
Objective: Our goal was to determine the association of maternal intrapartum antibiotics and early neonatal morbidity and mortality.
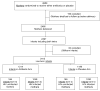
Methods: We performed secondary analysis of data from a multisite randomized, placebo-controlled clinical trial of antibiotics to prevent chorioamnionitis-associated mother-to-child transmission of HIV-1 and preterm birth in sub-Saharan Africa. Early neonatal morbidity and mortality were analyzed. In an intention-to-treat (ITT) analysis, infants born to women randomly assigned to antibiotics or placebo were compared. In addition, non-ITT analysis was performed because some women received nonstudy antibiotics for various clinical indications.
Results: Overall, 2659 pregnant women were randomly assigned. Of these, 2466 HIV-1-infected and HIV-1-uninfected women delivered 2413 live born and 84 stillborn infants. In the ITT analysis, there were no significant associations between exposure to antibiotics and early neonatal outcomes. Non-ITT analyses showed more illness at birth (11.2% vs 8.6%, P = .03) and more admissions to the special care infant unit (12.6% vs 9.8%, P = .04) among infants exposed to maternal intrapartum antibiotics than among unexposed infants. Additional analyses revealed greater early neonatal morbidity and mortality among infants of mothers who received nonstudy antibiotics than of mothers who received study antibiotics.
Conclusions: There is no association between intrapartum exposure to antibiotics and early neonatal morbidity or mortality. The associations observed in non-ITT analyses are most likely the result of women with peripartum illnesses being more likely to receive nonstudy antibiotics.
Conflict of interest statement
Figures
References
-
- World Health Organization. Neonatal and perinatal mortality: Country, regional and global estimates. WHO; Geneva: 2006.
-
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 22. New York: McGraw-Hill; 2006. pp. 855–880.
-
- Glasgow TS, Young PC, Wallin J, et al. Association of Intrapartum Antibiotic Exposure and Late-Onset Serious Bacterial Infections in Infants. Pediatrics. 2005;116 (3):696–702. - PubMed
-
- Towers CV, Carr MH, Padilla G, Asrat T. Potential consequences of widespread use of ampicillin. Am J Obstet Gynecol. 1998;179:879–883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical