Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance
- PMID: 19564370
- PMCID: PMC2737866
- DOI: 10.1128/AAC.00657-09
Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance
Abstract
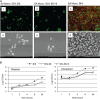
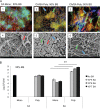
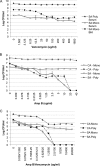
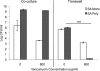
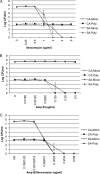
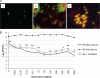
Candida albicans readily forms biofilms on the surface on indwelling medical devices, and these biofilms serve as a source of local and systemic infections. It is estimated that 27% of nosocomial C. albicans bloodstream infections are polymicrobial, with Staphylococcus aureus as the third most common organism isolated in conjunction with C. albicans. We tested whether S. aureus and C. albicans are able to form a polymicrobial biofilm. Although S. aureus formed poor monoculture biofilms in serum, it formed a substantial polymicrobial biofilm in the presence of C. albicans. In terms of architecture, S. aureus formed microcolonies on the surface of the biofilm, with C. albicans serving as the underlying scaffolding. In addition, S. aureus matrix staining revealed a different phenotype in polymicrobial versus monomicrobial biofilms, suggesting that S. aureus may become coated in the matrix secreted by C. albicans. S. aureus resistance to vancomycin was enhanced within the polymicrobial biofilm, required viable C. albicans, and was in part mediated by C. albicans matrix. However, the growth or sensitivity to amphotericin B of C. albicans is not altered in the polymicrobial biofilm.
Figures
References
-
- Adam, B., G. S. Baillie, and L. J. Douglas. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344-349. - PubMed
-
- Al-Fattani, M. A., and L. J. Douglas. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999-1008. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

