The NanA neuraminidase of Streptococcus pneumoniae is involved in biofilm formation
- PMID: 19564377
- PMCID: PMC2738052
- DOI: 10.1128/IAI.00228-09
The NanA neuraminidase of Streptococcus pneumoniae is involved in biofilm formation
Abstract
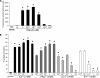
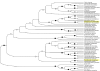
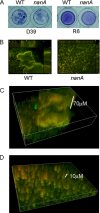
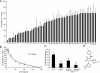
Streptococcus pneumoniae remains a major cause of bacteremia, pneumonia, and otitis media despite vaccines and effective antibiotics. The neuraminidase of S. pneumoniae, which catalyzes the release of terminal sialic acid residues from glycoconjugates, is involved in host colonization in animal models of infection and may provide a novel target for preventing pneumococcal infection. We demonstrate that the S. pneumoniae neuraminidase (NanA) cleaves sialic acid and show that it is involved in biofilm formation, suggesting an additional role in pathogenesis, and that it shares this property with the neuraminidase of Pseudomonas aeruginosa even though we show that the two enzymes are phylogenetically divergent. Using an in vitro model of biofilm formation incorporating human airway epithelial cells, we demonstrate that small-molecule inhibitors of NanA block biofilm formation and may provide a novel target for preventative therapy. This work highlights the role played by the neuraminidase in pathogenesis and represents an important step in drug development for prevention of colonization of the respiratory tract by this important pathogen.
Figures
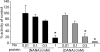
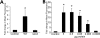
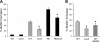
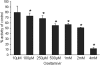
Similar articles
-
Pneumococcal neuraminidase A: an essential upper airway colonization factor for Streptococcus pneumoniae.Mol Oral Microbiol. 2012 Aug;27(4):270-83. doi: 10.1111/j.2041-1014.2012.00658.x. Mol Oral Microbiol. 2012. PMID: 22759312
-
Pneumococcal Neuraminidase A (NanA) Promotes Biofilm Formation and Synergizes with Influenza A Virus in Nasal Colonization and Middle Ear Infection.Infect Immun. 2017 Mar 23;85(4):e01044-16. doi: 10.1128/IAI.01044-16. Print 2017 Apr. Infect Immun. 2017. PMID: 28096183 Free PMC article.
-
Neuraminidase A-Exposed Galactose Promotes Streptococcus pneumoniae Biofilm Formation during Colonization.Infect Immun. 2016 Sep 19;84(10):2922-32. doi: 10.1128/IAI.00277-16. Print 2016 Oct. Infect Immun. 2016. PMID: 27481242 Free PMC article.
-
Pneumococcal modification of host sugars: a major contributor to colonization of the human airway?Mol Oral Microbiol. 2010 Feb;25(1):15-24. doi: 10.1111/j.2041-1014.2009.00564.x. Mol Oral Microbiol. 2010. PMID: 20331791 Review.
-
Haemophilus influenzae and Streptococcus pneumoniae: living together in a biofilm.Pathog Dis. 2013 Nov;69(2):114-26. doi: 10.1111/2049-632X.12073. Epub 2013 Sep 10. Pathog Dis. 2013. PMID: 23913525 Review.
Cited by
-
Unified theory of bacterial sialometabolism: how and why bacteria metabolize host sialic acids.ISRN Microbiol. 2013 Jan 15;2013:816713. doi: 10.1155/2013/816713. Print 2013. ISRN Microbiol. 2013. PMID: 23724337 Free PMC article.
-
Diverse Mechanisms of Protective Anti-Pneumococcal Antibodies.Front Cell Infect Microbiol. 2022 Jan 28;12:824788. doi: 10.3389/fcimb.2022.824788. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35155281 Free PMC article. Review.
-
Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a. A new paradigm in respiratory infection.Am J Respir Crit Care Med. 2014 Jul 15;190(2):196-207. doi: 10.1164/rccm.201311-2110OC. Am J Respir Crit Care Med. 2014. PMID: 24941423 Free PMC article.
-
Abrogation of neuraminidase reduces biofilm formation, capsule biosynthesis, and virulence of Porphyromonas gingivalis.Infect Immun. 2012 Jan;80(1):3-13. doi: 10.1128/IAI.05773-11. Epub 2011 Oct 24. Infect Immun. 2012. PMID: 22025518 Free PMC article.
-
The Sialidase NanS Enhances Non-TcsL Mediated Cytotoxicity of Clostridium sordellii.Toxins (Basel). 2016 Jun 17;8(6):189. doi: 10.3390/toxins8060189. Toxins (Basel). 2016. PMID: 27322322 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

