Bicarbonate Induces Vibrio cholerae virulence gene expression by enhancing ToxT activity
- PMID: 19564378
- PMCID: PMC2738005
- DOI: 10.1128/IAI.00409-09
Bicarbonate Induces Vibrio cholerae virulence gene expression by enhancing ToxT activity
Erratum in
- Infect Immun. 2009 Nov;77(11):5202
Abstract
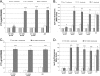
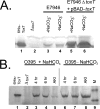
Vibrio cholerae is a gram-negative bacterium that is the causative agent of cholera, a severe diarrheal illness. The two biotypes of V. cholerae O1 capable of causing cholera, classical and El Tor, require different in vitro growth conditions for induction of virulence gene expression. Growth under the inducing conditions or infection of a host initiates a complex regulatory cascade that results in production of ToxT, a regulatory protein that directly activates transcription of the genes encoding cholera toxin (CT), toxin-coregulated pilus (TCP), and other virulence genes. Previous studies have shown that sodium bicarbonate induces CT expression in the V. cholerae El Tor biotype. However, the mechanism for bicarbonate-mediated CT induction has not been defined. In this study, we demonstrate that bicarbonate stimulates virulence gene expression by enhancing ToxT activity. Both the classical and El Tor biotypes produce inactive ToxT protein when they are cultured statically in the absence of bicarbonate. Addition of bicarbonate to the culture medium does not affect ToxT production but causes a significant increase in CT and TCP expression in both biotypes. Ethoxyzolamide, a potent carbonic anhydrase inhibitor, inhibits bicarbonate-mediated virulence induction, suggesting that conversion of CO(2) into bicarbonate by carbonic anhydrase plays a role in virulence induction. Thus, bicarbonate is the first positive effector for ToxT activity to be identified. Given that bicarbonate is present at high concentration in the upper small intestine where V. cholerae colonizes, bicarbonate is likely an important chemical stimulus that V. cholerae senses and that induces virulence during the natural course of infection.
Figures




References
-
- Cash, R. A., S. I. Music, J. P. Libonati, M. J. Snyder, R. P. Wenzel, and R. B. Hornick. 1974. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J. Infect. Dis. 12945-52. - PubMed
-
- Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23323-331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

