LuxS promotes biofilm maturation and persistence of nontypeable haemophilus influenzae in vivo via modulation of lipooligosaccharides on the bacterial surface
- PMID: 19564381
- PMCID: PMC2738029
- DOI: 10.1128/IAI.00320-09
LuxS promotes biofilm maturation and persistence of nontypeable haemophilus influenzae in vivo via modulation of lipooligosaccharides on the bacterial surface
Abstract
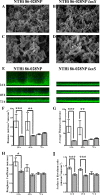
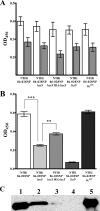
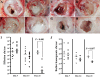
Nontypeable Haemophilus influenzae (NTHI) is an extremely common airway commensal which can cause opportunistic infections that are usually localized to airway mucosal surfaces. During many of these infections, NTHI forms biofilm communities that promote persistence in vivo. For many bacterial species, density-dependent quorum-signaling networks can affect biofilm formation and/or maturation. Mutation of luxS, a determinant of the autoinducer 2 (AI-2) quorum signal pathway, increases NTHI virulence in the chinchilla model for otitis media infections. For example, bacterial counts in middle-ear fluids and the severity of the host inflammatory response were increased in luxS mutants compared with parental strains. As these phenotypes are consistent with those that we have observed for biofilm-defective NTHI mutants, we hypothesized that luxS may affect NTHI biofilms. A luxS mutant was generated using the well-characterized NTHI 86-028NP strain and tested to determine the effects of the mutation on biofilm phenotypes in vitro and bacterial persistence and disease severity during experimental otitis media. Quantitation of the biofilm structure by confocal microscopy and COMSTAT analysis revealed significantly reduced biomass for NTHI 86-028NP luxS biofilms, which was restored by a soluble mediator in NTHI 86-028NP supernatants. Analysis of lipooligosaccharide moieties using an enzyme-linked immunosorbent assay and immunoblotting showed decreased levels of biofilm-associated glycoforms in the NTHI 86-028NP luxS strain. Infection studies showed that NTHI 86-028NP luxS had a significant persistence defect in vivo during chronic otitis media infection. Based on these data, we concluded that a luxS-dependent soluble mediator modulates the composition of the NTHI lipooligosaccharides, resulting in effects on biofilm maturation and bacterial persistence in vivo.
Figures
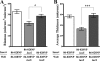

References
-
- Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. Randle, A. Martin, Z. Li, R. Goldstein, and E. K. Schweda. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 1008898-8903. - PMC - PubMed
-
- Campagnari, A. A., S. M. Spinola, A. J. Lesse, Y. Abu Kwaik, R. E. Mandrell, and M. Apicella. 1990. Lipooligosaccharide epitopes shared among Gram-negative non-enteric mucosal pathogens. Microb. Pathog. 8353-362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

