Postoperative surveillance recommendations for early stage colon cancer based on results from the clinical outcomes of surgical therapy trial
- PMID: 19564531
- PMCID: PMC2720080
- DOI: 10.1200/JCO.2008.20.7050
Postoperative surveillance recommendations for early stage colon cancer based on results from the clinical outcomes of surgical therapy trial
Abstract
Purpose: Intensive postoperative surveillance is associated with improved survival and recommended for patients with late stage (stage IIB and III) colon cancer. We hypothesized that stage I and IIA colon cancer patients would experience similar benefits.
Patients and methods: Secondary analysis of data from the Clinical Outcomes of Surgical Therapy trial was performed by analyzing results according to TNM stage; early (stage I and IIA, 537 patients) and late (stage IIB and III, 254 patients) stage disease. Five-year recurrence rates were higher in patients with late (35.7%) versus early stage disease (9.5%). Early and late stage salvage rates, recurrence patterns and methods of first detection were compared by chi(2) test.
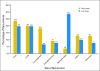
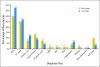
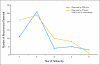
Results: Salvage rates for early- and late-stage disease patients with recurrence were the same (35.9% v 37%; P = .9, respectively). Median survival after second surgery after recurrence was 51.2 and 35.8 months for early- and late-stage patients, respectively. Single sites of first recurrence did not significantly differ between early and late stage, but multiple sites of recurrence occurred less often in early-stage patients (3.6% v 28.6%, for early v late, respectively; P < .001). METHODS of first detection of recurrence were not significantly different: carcinoembryonic antigen (29.1% v 37.4%), computed tomography scan (23.6% v 26.4%), chest x-ray (7.3% v 12.1%), and colonoscopy (12.7% v 8.8%), for early versus late stage disease, respectively.
Conclusion: Patients with early-stage colon cancer have similar sites of recurrence, and receive similar benefit from postrecurrence therapy as late-stage patients; implementation of surveillance guidelines for early-stage patients is appropriate.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Caution is required before recommending routine carcinoembryonic antigen and imaging follow-up for patients with early-stage colon cancer.J Clin Oncol. 2009 Dec 20;27(36):e279-80; author reply e281. doi: 10.1200/JCO.2009.25.6156. Epub 2009 Nov 9. J Clin Oncol. 2009. PMID: 19901127 No abstract available.
References
-
- Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;(1) CD002200. - PubMed
-
- Anthony T, Simmang C, Hyman N, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47:807–817. - PubMed
-
- Desch CE, Benson AB, III, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. - PubMed
-
- Arriola E, Navarro M, Pares D, et al. Imaging techniques contribute to increased surgical rescue of relapse in the follow-up of colorectal cancer. Dis Colon Rectum. 2006;49:478–484. - PubMed
-
- Rodriguez-Moranta F, Salo J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: A prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386–393. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

