Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225
- PMID: 19564532
- PMCID: PMC2720082
- DOI: 10.1200/JCO.2008.19.9109
Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225
Abstract
Purpose: To investigate the feasibility of intensity-modulated radiation therapy (IMRT) with or without chemotherapy, and to assess toxicities, failure patterns, and survivals in patients with nasopharyngeal carcinoma (NPC).
Patients and methods: Radiation consisted of 70 Gy given to the planning target volumes of primary tumor plus any N+ disease and 59.4 Gy given to subclinical disease, delivered over 33 treatment days. Patients with stage T2b or greater or with N+ disease also received concurrent cisplatin (100 mg/m(2)) on days 1, 22, and 43 followed by adjuvant cisplatin (80 mg/m(2)) on day 1; fluorouracil (1,000 mg/m(2)/d) on days 1 through 4 administered every 4 weeks for three cycles. Tumor, clinical status, and acute/late toxicities were assessed. The primary objective was to test the transportability of IMRT to a multi-institutional setting.
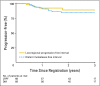
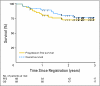
Results: Between February 2003 and November 2005, 68 patients with stages I through IVB NPC (of which 93.8% were WHO types 2 and 3) were enrolled. Prescribed IMRT (target delineation) was given to 83.8%, whereas 64.9% received chemotherapy per protocol. The estimated 2-year local progression-free (PF), regional PF, locoregional PF, and distant metastasis-free rates were 92.6%, 90.8%, 89.3%, and 84.7%, respectively. The estimated 2-year PF and overall survivals were 72.7% and 80.2%, respectively. Acute grade 4 mucositis occurred in 4.4%, and the worst late grade 3 toxicities were as follows: esophagus, 4.7%; mucous membranes, 3.1%; and xerostomia, 3.1%. The rate of grade 2 xerostomia at 1 year from start of IMRT was 13.5%. Only two patients complained of grade 3 xerostomia, and none had grade 4 xerostomia.
Conclusion: It was feasible to transport IMRT with or without chemotherapy in the treatment of NPC to a multi-institutional setting with 90% LRPF rate reproducing excellent reports from single institutions. Minimal grade 3 and lack of grade 4 xerostomia were encouraging.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Yu MC. Diet and nasopharyngeal carcinoma. FEMS Microbiol Immunol. 1990;2:235–242. - PubMed
-
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. - PubMed
-
- Langendijk JA, Leemans CR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: A meta-analysis of the published literature. J Clin Oncol. 2004;22:4604–4612. - PubMed
-
- Chu AM, Flynn MB, Achino E, et al. Irradiation of nasopharyngeal carcinoma: Correlations with treatment factors and stage. Int J Radiat Oncol Biol Phys. 1984;10:2241–2249. - PubMed
-
- Hoppe RT, Goffinet DR, Bagshaw MA. Carcinoma of the nasopharynx: Eighteen years' experience with megavoltage radiation therapy. Cancer. 1976;37:2605–2612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

