Treatment of myelodysplastic syndrome patients with erythropoietin with or without granulocyte colony-stimulating factor: results of a prospective randomized phase 3 trial by the Eastern Cooperative Oncology Group (E1996)
- PMID: 19564636
- PMCID: PMC2746469
- DOI: 10.1182/blood-2009-03-211797
Treatment of myelodysplastic syndrome patients with erythropoietin with or without granulocyte colony-stimulating factor: results of a prospective randomized phase 3 trial by the Eastern Cooperative Oncology Group (E1996)
Abstract
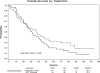
This phase 3 prospective randomized trial evaluated the efficacy and long-term safety of erythropoietin (EPO) with or without granulocyte colony-stimulating factor plus supportive care (SC; n = 53) versus SC alone (n = 57) for the treatment of anemic patients with lower-risk myelodysplastic syndromes. The response rates in the EPO versus SC alone arms were 36% versus 9.6%, respectively, at the initial treatment step, 47% in the EPO arm, including subsequent steps. Responding patients had significantly lower serum EPO levels (45% vs 5% responses for levels < 200 mU/mL vs > or = 200 mU/mL) and improvement in multiple quality-of-life domains. With prolonged follow-up (median, 5.8 years), no differences were found in overall survival of patients in the EPO versus SC arms (median, 3.1 vs 2.6 years) or in the incidence of transformation to acute myeloid leukemia (7.5% and 10.5% patients, respectively). Increased survival was demonstrated for erythroid responders versus nonresponders (median, 5.5 vs 2.3 years). Flow cytometric analysis showed that the percentage of P-glycoprotein(+) CD34(+) marrow blasts was positively correlated with longer overall survival. In comparison with SC alone, patients receiving EPO with or without granulocyte colony-stimulating factor plus SC had improved erythroid responses, similar survival, and incidence of acute myeloid leukemia transformation.
Figures



Comment in
-
Out of this nettle, danger, we must pluck this flower, safety.Blood. 2009 Sep 17;114(12):2364-5. doi: 10.1182/blood-2009-07-231779. Blood. 2009. PMID: 19762499 No abstract available.
References
-
- Greenberg PL. The myelodysplastic syndromes. In: Hoffman R, Benz E, Shattil S, editors. Hematology: Basic Principles and Practice. 3rd Ed. New York, NY: Churchill Livingstone; 1999. pp. 1106–1129.
-
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. - PubMed
-
- Greenberg PL, Attar E, Battiwalla M, et al. NCCN Practice Guidelines for Myelodysplastic Syndromes, Version 1. 2009. J Nat Comp Canc Network. 2008;6(1):902–925.
-
- Stone RM, Bernstein SH, Demetri G, et al. Treatment with recombinant human erythropoietin in patients with myelodysplastic syndromes. Leuk Res. 1994;18(10):769–776. - PubMed
-
- Rose EH, Abels RI, Nelson RA, et al. The use of r-HuEPO in the treatment of anaemia related to myelodysplasia (MDS). Br J Haematol. 1995;89(4):831–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA07190/CA/NCI NIH HHS/United States
- U10 CA013650/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- CA13650/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA080775/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- CA80775/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- U10 CA007190/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

