RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack
- PMID: 19564897
- PMCID: PMC2694982
- DOI: 10.1371/journal.pbio.1000139
RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack
Abstract
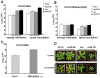
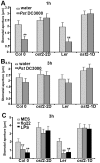
Pathogen perception by the plant innate immune system is of central importance to plant survival and productivity. The Arabidopsis protein RIN4 is a negative regulator of plant immunity. In order to identify additional proteins involved in RIN4-mediated immune signal transduction, we purified components of the RIN4 protein complex. We identified six novel proteins that had not previously been implicated in RIN4 signaling, including the plasma membrane (PM) H(+)-ATPases AHA1 and/or AHA2. RIN4 interacts with AHA1 and AHA2 both in vitro and in vivo. RIN4 overexpression and knockout lines exhibit differential PM H(+)-ATPase activity. PM H(+)-ATPase activation induces stomatal opening, enabling bacteria to gain entry into the plant leaf; inactivation induces stomatal closure thus restricting bacterial invasion. The rin4 knockout line exhibited reduced PM H(+)-ATPase activity and, importantly, its stomata could not be re-opened by virulent Pseudomonas syringae. We also demonstrate that RIN4 is expressed in guard cells, highlighting the importance of this cell type in innate immunity. These results indicate that the Arabidopsis protein RIN4 functions with the PM H(+)-ATPase to regulate stomatal apertures, inhibiting the entry of bacterial pathogens into the plant leaf during infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. - PubMed
-
- Nurnberger T, Kemmerling B. Receptor protein kinases–pattern recognition receptors in plant immunity. Trends Plant Sci. 2006;11:519–522. - PubMed
-
- Zhou JM, Chai J. Plant pathogenic bacterial type III effectors subdue host responses. Curr Opin Microbiol. 2008;11:179–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

