Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus
- PMID: 19564903
- PMCID: PMC2696347
- DOI: 10.1371/journal.pbio.1000140
Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus
Erratum in
- PLoS Biol. 2010;8(10). doi: 10.1371/annotation/5e28240c-6186-43eb-b319-79c391d9468a
- PLoS Biol. 2010;8(10). doi: 10.1371/annotation/d0e5ef6f-d08b-474a-8fde-aeebeee7369d
Abstract
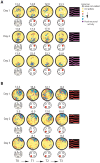
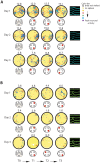
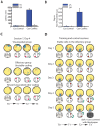
A key question in the analysis of hippocampal memory relates to how attention modulates the encoding and long-term retrieval of spatial and nonspatial representations in this region. To address this question, we recorded from single cells over a period of 5 days in the CA1 region of the dorsal hippocampus while mice acquired one of two goal-oriented tasks. These tasks required the animals to find a hidden food reward by attending to either the visuospatial environment or a particular odor presented in shifting spatial locations. Attention to the visuospatial environment increased the stability of visuospatial representations and phase locking to gamma oscillations--a form of neuronal synchronization thought to underlie the attentional mechanism necessary for processing task-relevant information. Attention to a spatially shifting olfactory cue compromised the stability of place fields and increased the stability of reward-associated odor representations, which were most consistently retrieved during periods of sniffing and digging when animals were restricted to the cup locations. Together, these results suggest that attention selectively modulates the encoding and retrieval of hippocampal representations by enhancing physiological responses to task-relevant information.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Role of the hippocampus in goal-oriented tasks requiring retrieval of spatial versus non-spatial information.Neurobiol Learn Mem. 2010 May;93(4):581-8. doi: 10.1016/j.nlm.2010.02.006. Epub 2010 Mar 3. Neurobiol Learn Mem. 2010. PMID: 20206279
-
Motivational states activate distinct hippocampal representations to guide goal-directed behaviors.Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10805-10. doi: 10.1073/pnas.0903259106. Epub 2009 Jun 15. Proc Natl Acad Sci U S A. 2009. PMID: 19528659 Free PMC article.
-
Increased attention to spatial context increases both place field stability and spatial memory.Neuron. 2004 Apr 22;42(2):283-95. doi: 10.1016/s0896-6273(04)00192-8. Neuron. 2004. PMID: 15091343
-
What is remembered? Role of attention on the encoding and retrieval of hippocampal representations.J Physiol. 2009 Jun 15;587(Pt 12):2837-54. doi: 10.1113/jphysiol.2009.172445. J Physiol. 2009. PMID: 19525568 Free PMC article. Review.
-
Parallel processing across neural systems: implications for a multiple memory system hypothesis.Neurobiol Learn Mem. 2004 Nov;82(3):278-98. doi: 10.1016/j.nlm.2004.07.007. Neurobiol Learn Mem. 2004. PMID: 15464410 Review.
Cited by
-
Attention Stabilizes Representations in the Human Hippocampus.Cereb Cortex. 2016 Feb;26(2):783-796. doi: 10.1093/cercor/bhv041. Epub 2015 Mar 12. Cereb Cortex. 2016. PMID: 25766839 Free PMC article.
-
Feature selectivity is stable in primary visual cortex across a range of spatial frequencies.Sci Rep. 2018 Oct 16;8(1):15288. doi: 10.1038/s41598-018-33633-2. Sci Rep. 2018. PMID: 30327571 Free PMC article.
-
Flexible weighting of diverse inputs makes hippocampal function malleable.Neurosci Lett. 2018 Jul 27;680:13-22. doi: 10.1016/j.neulet.2017.05.063. Epub 2017 Jun 3. Neurosci Lett. 2018. PMID: 28587901 Free PMC article. Review.
-
Synchronous neural activity and memory formation.Curr Opin Neurobiol. 2010 Apr;20(2):150-5. doi: 10.1016/j.conb.2010.02.006. Epub 2010 Mar 18. Curr Opin Neurobiol. 2010. PMID: 20303255 Free PMC article. Review.
-
Differential effect of sleep deprivation on place cell representations, sleep architecture, and memory in young and old mice.Cell Rep. 2021 Jun 15;35(11):109234. doi: 10.1016/j.celrep.2021.109234. Cell Rep. 2021. PMID: 34133936 Free PMC article.
References
-
- Ferbinteanu J, Kennedy PJ, Shapiro ML. Episodic memory–from brain to mind. Hippocampus. 2006;16:691–703. - PubMed
-
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16:716–729. - PubMed
-
- Fernandes MA, Moscovitch M, Ziegler M, Grady C. Brain regions associated with successful and unsuccessful retrieval of verbal episodic memory as revealed by divided attention. Neuropsychologia. 2005;43:1115–1127. - PubMed
-
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

