Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer
- PMID: 19565923
- PMCID: PMC7122391
- DOI: 10.1007/978-1-59745-561-9_30
Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer
Abstract
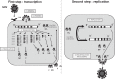
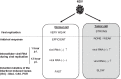
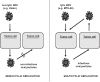
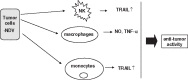
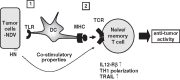
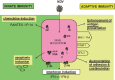
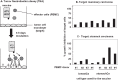
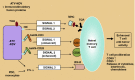
This review deals with the avian paramyxovirus Newcastle disease virus (NDV) and describes properties that explain its oncolytic activity, its tumor-selective replication behavior, and its immune-stimulatory capacity with human cells. The strong interferon response of normal cells upon contact with NDV appears to be the basis for the good tolerability of the virus in cancer patients and for its immune stimulatory properties, whereas the weak interferon response of tumor cells explains the tumor selectivity of replication and oncolysis. Various concepts for the use of this virus for cancer treatment are pointed out and results from clinical studies are summarized. Reverse genetics technology has made it possible recently to clone the genome and to introduce new foreign genes thus generating new recombinant viruses. These can, in the future, be used to transfer new therapeutic genes into tumors and also to immunize against new emerging pathogens. The modular nature of gene transcription, the undetectable rate of recombination, and the lack of a DNA phase in the replication cycle make NDV a suitable candidate for the rational design of a safe and stable vaccine and gene therapy vector.
Figures


Similar articles
-
Oncolytic therapy and gene therapy for cancer: recent advances in antitumor effects of Newcastle disease virus.Discov Med. 2020 Jul-Aug;30(159):39-48. Discov Med. 2020. PMID: 33357361 Review.
-
The Application of Newcastle Disease Virus (NDV): Vaccine Vectors and Tumor Therapy.Viruses. 2024 May 30;16(6):886. doi: 10.3390/v16060886. Viruses. 2024. PMID: 38932177 Free PMC article. Review.
-
Oncolytic Newcastle disease virus as a prospective anti-cancer therapy. A biologic agent with potential to break therapy resistance.Expert Opin Biol Ther. 2015;15(12):1757-71. doi: 10.1517/14712598.2015.1088000. Epub 2015 Oct 5. Expert Opin Biol Ther. 2015. PMID: 26436571 Review.
-
Design and Production of Newcastle Disease Virus for Intratumoral Immunomodulation.Methods Mol Biol. 2020;2058:133-154. doi: 10.1007/978-1-4939-9794-7_9. Methods Mol Biol. 2020. PMID: 31486036 Free PMC article.
-
Genetic Modification of Oncolytic Newcastle Disease Virus for Cancer Therapy.J Virol. 2016 May 12;90(11):5343-5352. doi: 10.1128/JVI.00136-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27009956 Free PMC article.
Cited by
-
Enhanced Oncolytic Potential of Engineered Newcastle Disease Virus Lasota Strain through Modification of Its F Protein Cleavage Site.Microorganisms. 2024 Oct 8;12(10):2029. doi: 10.3390/microorganisms12102029. Microorganisms. 2024. PMID: 39458338 Free PMC article.
-
Oncolytic viruses in the treatment of cancer: a review of current strategies.Pathol Oncol Res. 2012 Oct;18(4):771-81. doi: 10.1007/s12253-012-9548-2. Epub 2012 Jun 20. Pathol Oncol Res. 2012. PMID: 22714538 Review.
-
Inhibitory receptors as targets for cancer immunotherapy.Eur J Immunol. 2015 Jul;45(7):1892-905. doi: 10.1002/eji.201344413. Eur J Immunol. 2015. PMID: 26018646 Free PMC article. Review.
-
Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions.Future Microbiol. 2012 Mar;7(3):347-67. doi: 10.2217/fmb.12.4. Future Microbiol. 2012. PMID: 22393889 Free PMC article. Review.
-
Exploring the Prospects of Engineered Newcastle Disease Virus in Modern Vaccinology.Viruses. 2020 Apr 16;12(4):451. doi: 10.3390/v12040451. Viruses. 2020. PMID: 32316317 Free PMC article. Review.
References
-
- Chlichlia K., Schirrmacher V., Sandaltzopoulos R. Cancer immunotherapy: battling tumors with gene vaccines. Curr. Med. Chem. Anti-inflammatory Anti-allergy Agents. 2005;4:353–365. doi: 10.2174/1568014054546290. - DOI
-
- Sinkovics J., Horvatz J. New developments in the virus therapy of cancer: a historical review. Intervirology. 1993;36:193–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

