ENDOR spectroscopy shows that guanine N1 binds to [4Fe-4S] cluster II of the S-adenosylmethionine-dependent enzyme MoaA: mechanistic implications
- PMID: 19566093
- PMCID: PMC2757093
- DOI: 10.1021/ja903978u
ENDOR spectroscopy shows that guanine N1 binds to [4Fe-4S] cluster II of the S-adenosylmethionine-dependent enzyme MoaA: mechanistic implications
Abstract
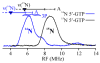
The S-adenosylmethionine-dependent enzyme MoaA, in concert with MoaC, catalyzes the first step of molybdenum cofactor biosynthesis, the conversion of guanosine 5'-triphosphate (5'-GTP) into precursor Z. A published X-ray crystal structure of MoaA with the substrate 5'-GTP revealed that the substrate might be bound to the unique iron of one of two 4Fe-4S clusters through either or both the amino and N1 nitrogen nuclei. Use of 35 GHz continuous-wave ENDOR spectroscopy of MoaA with unlabeled and (15)N-labeled substrate and a reduced [4Fe-4S](+) cluster now demonstrates that only one nitrogen nucleus is bound to the cluster. Experiments with the substrate analogue inosine 5'-triphosphate further demonstrate that it is the N1 nitrogen that binds. Two of the more distant nitrogen nuclei have also been detected by 35 GHz pulsed ENDOR spectroscopy, allowing a rough approximation of their distances from the cluster to be calculated. Combining this information with the crystal structure, we propose that the guanine base adopts the enol tautomer as N1 binds to Fe4 and the O6-H hydroxyl group forms a hydrogen bond with S4 of the 4Fe-4S cluster, and that this binding-induced tautomerization may have important mechanistic ramifications.
Figures


References
-
- Wuebbens MM, Rajagopalan KV. J Biol Chem. 1995;270:1082–1087. - PubMed
-
- Werst MM, Davoust CE, Hoffman BM. J Am Chem Soc. 1991;113:1533–1538.
-
- Davoust CE, Doan PE, Hoffman BM. J Magn Reson. 1996;119:38–44.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

