Morphological characterization of pecteneal hyalocytes in the developing quail retina
- PMID: 19566699
- PMCID: PMC2750761
- DOI: 10.1111/j.1469-7580.2009.01117.x
Morphological characterization of pecteneal hyalocytes in the developing quail retina
Abstract
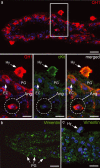
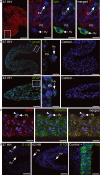
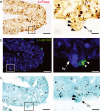
The periphery of the vitreous body contains a population of cells termed hyalocytes. Despite the existence for more than one century of publications devoted to the pecten oculi, a convoluted coil of blood vessels that seems to be the primary source of nutrients for the avian avascular retina, little information can be found concerning the pecteneal hyalocytes. These cells are situated on the inner limiting membrane in close relationship with the convolute blood vessels. To characterize the origin and macrophagic activity of pecteneal hyalocytes, we have analysed two different stages of quail eye development using histochemistry and immunohistochemistry. Pecteneal hyalocytes express the QH1 epitope and cKit, confirming that these cells belong to the haematopoietic system. They also express vimentin, an intermediate filament protein present in cells of mesenchymal origin and very important for differentiation of fully active macrophages. However, similarly as described in porcine hyalocytes, pecteneal hyalocytes express the glial fibrillary acidic protein, a recognized neuroglial marker. Pecteneal hyalocytes did not express other neuroglial markers, such as glutamine synthetase or S100. Acidic phosphatase was activated and Lep100 was found in secondary lysosomes, confirming phagocytic activity of pecteneal hyalocytes during ocular development. Pecteneal hyalocytes strongly react with RCA-I, WFA, WGA, PNA, SNA, LEA and SBA lectins, whereas other avian macrophages from thymus and the bursa of Fabricius did not bind PNA, SNA and LEA lectins. Interestingly, WGA lectin reacts with all kinds of avian macrophages, including pecteneal hyalocytes, probably reflecting the specific binding of WGA to components of the phagocytic and endocytic pathways. In conclusion, pecteneal hyalocytes are a special subtype of blood-borne macrophages that express markers not specifically associated with the haematopoietic system.
Figures


References
-
- Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signalling in mammalian melanocytes. J Invest Dermatol. 2006;126:1102–1110. - PubMed
-
- Alroy J, Goyal V, Skutelsky E. Lectin histochemistry of mammalian endothelium. Histochemistry. 1987;86:603–607. - PubMed
-
- Arrequi C, Barra HS, Landa CA. Peanut agglutinin binding glycoproteins in the chick retina: their presence in Muller glia cells. J Neurosci Res. 1992;31:532–542. - PubMed
-
- Bawa SR, YashRoy RC. Structure and function of vulture pecten. Acta Anat. 1974;89:473–480. - PubMed
-
- Benes P, Macecková V, Zdráhal Z, et al. Role of vimentin in regulation of monocyte/macrophage differentiation. Differentiation. 2006;74:265–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

